During Gain Therapeutics R&D Day, Samuel Broder, MD shares his perspective on Neuroscience and Beyond. The title of his presentation was “The Role of Allosteric Drug Discovery in Targeting Undruggable Proteins in Oncology”.
He discusses about Harnessing Computational Technology and Allosteric Modulators to Drug the Undruggable. Stressing on the opportunities for the Gain Therapeutics Platform of Site-Directed Enzyme Enhancement Therapy (SEE-Tx™) platform and related proprietary technologies.
See full video here:
Here is the Transcript of the Presentation by Samuel Broder, MD
Thank you very much for the introduction and I’m very pleased to be here and honored to participate in the research and development programs of Gain Therapeutics. I’m especially honored to follow the wonderful presentation that you’ve just heard.
My topic today is the role of allosteric drug discovery in targeting undruggable proteins in oncology. What I’d like to do is focus on opportunities for the Gain Therapeutics platform of Site-Directed Enzyme Enhancement Therapy, that platform and related proprietary technologies in the overall development of new therapeutic interactions.
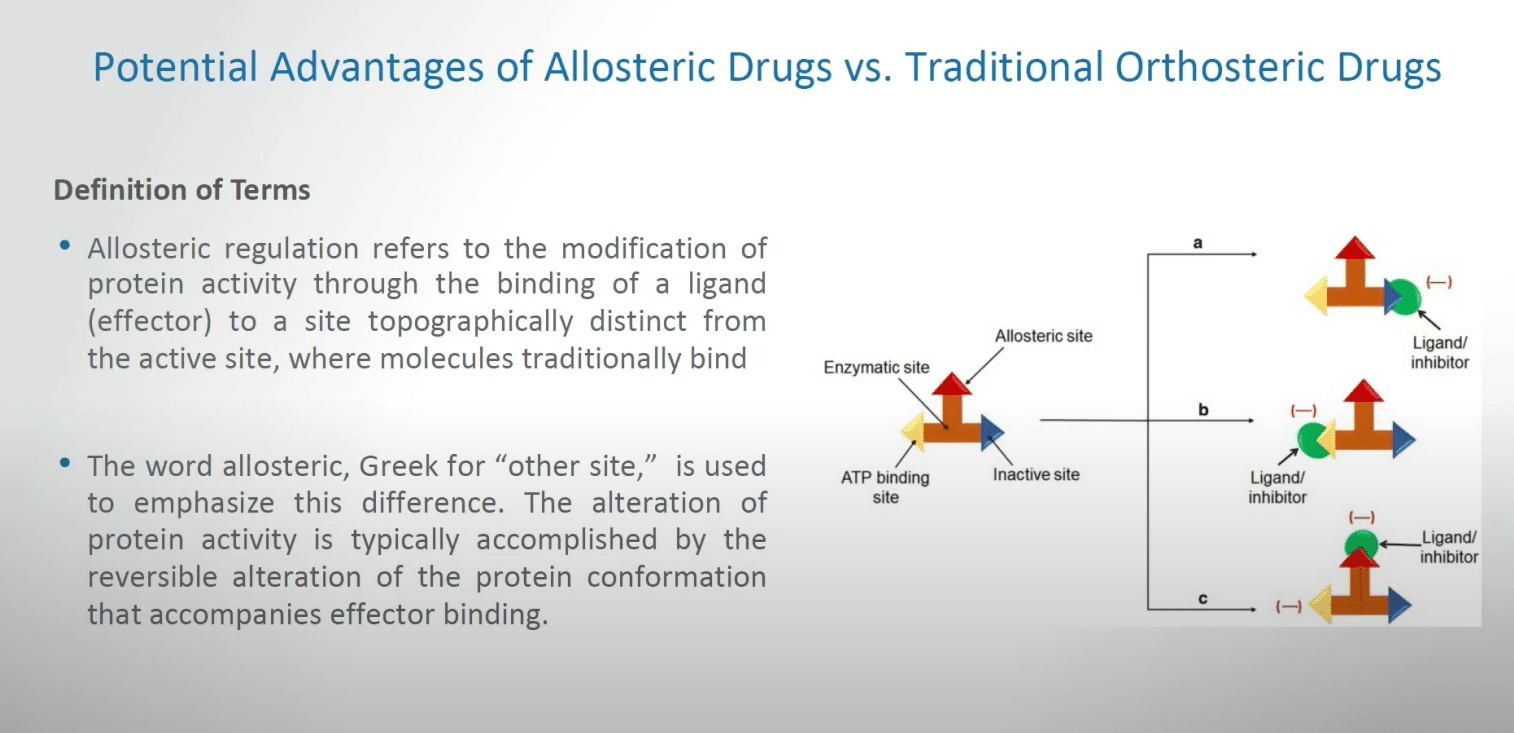
We heard a little bit about the potential advantages of allosteric drugs versus traditional orthosteric drugs. What I’d like to do is briefly recapitulate a definition of terms.
Allosteric regulation refers to the modification of protein activity through the binding of a ligand, sometimes called an effector, to a site topographically distinct from the active site where molecules traditionally bind in either the setting of enzymes or in certain cases receptors. The word allosteric, as you’ve heard, really arises from the Greek for ‘other site’ and is used to emphasize this difference.
The alteration of protein activity is typically accomplished by the reversible alteration of the protein confirmation that accompanies effector binding, and you’ve heard some wonderful introductions along this line. There is a little bit of a schematic on the right there, but you’ve already heard a number of the issues in how this works.
I’d like to give you my own analog, my own analogy of what allosteric inhibition really is, and all analogies are fraught with potential issues, but this is how I would like to at least put on the table how we can allosteric inhibition.
Imagine that you have an extraordinarily bright light bulb on the second floor of your house and it’s on in a way that you can’t control. It has a switch which is difficult to get ahold of, and in any event has been wired in such a way that when you try to turn that switch off many other lights in the house go off. What an allosteric intervention could represent is essentially finding a dimmer switch, perhaps in your basement, that can exquisitely control the obnoxious light in discussion. So, in other words, you can modulate a function that you either wish to enhance or depress, but you can do so in a way that is at a distance from the functional portion of the target that you’re discussing.

There are potential advantages in allosteric drugs that we are talking about in medical oncology and they provide a very important new dimension.
I at this point might remind the audience that medical oncology and oncology drug related discoveries are an extraordinarily rapidly growing phase of the entire pharmaceutical development. There have been astonishing levels of public health and commercial advances in the field of medical oncology and we expect that to continue.
In fact, I could at this point briefly introduce the notion that President Biden has recently launched another program aimed at reducing the death rate from cancer by at least 50% in a reasonable period, and I personally believe that Gain and its platform can play a significant role in this goal. So let’s go over some potential advantages of allosteric drugs in medical oncology. Some of this will recapitulate what you’ve already heard.
There’s greater specificity and selectivity of activity. Targeting allosteric sites may provide greater specificity and selectivity of activity, allowing for a better efficacy and a better safety profile. I want to emphasize that selectivity is a key advantage. A protein’s active site can appear similar to other proteins in its family, making it difficult, sometimes in the extreme, to limit off-target and off-tumor effects. An allosteric site is more specific to an individual protein, potentially leading to safer and more efficacious medicines.
An allosteric intervention may modulate a protein’s activity in new ways. On modulator may shift a protein into a conformation that shuts it down entirely, while another, under the right conditions, may potentiate activity where such activity is desired, and any measure of activity in between.
Another key feature is that one can identify previously inaccessible drug targets. We’ve already heard the term undruggable. Allosteric drugs open the door to identifying drug targets that are inaccessible to traditional active inhibitors, and that’s a point that we need to keep in mind. Also, I believe this offers a new foundation for combining oncolytic agents. There’s a potential to achieve important additive and synergistic effects, and to prevent or overcome additional orthosteric anti-cancer agent resistance and so forth.

The need for novel kinase inhibitors is just one example of what may lie in store and the solutions that we may have. The future success in kinase drug discoveries, which is a very important component of modern medical oncology, will depend on finding new ways to identify chemically more diverse specific inhibitor with good physicochemical properties, and I might add good pharmacokinetic properties.
Finding compounds that are not already covered by intellectual property claims is also an important feature, and finding novel approaches to address resistance is also extremely important.
One of the problems that we face in medical oncology and therapeutics related to cancer interventions is that when patients have advanced disease, to a very large extent drug resistance can become an enormous problem. It is one of the reasons why when patients have advanced tumors, especially visceral advanced tumors, often they will die of their tumor unless there is a co-morbid event that takes them off first.
These are significant challenges that I believe allosteric approaches can benefit.
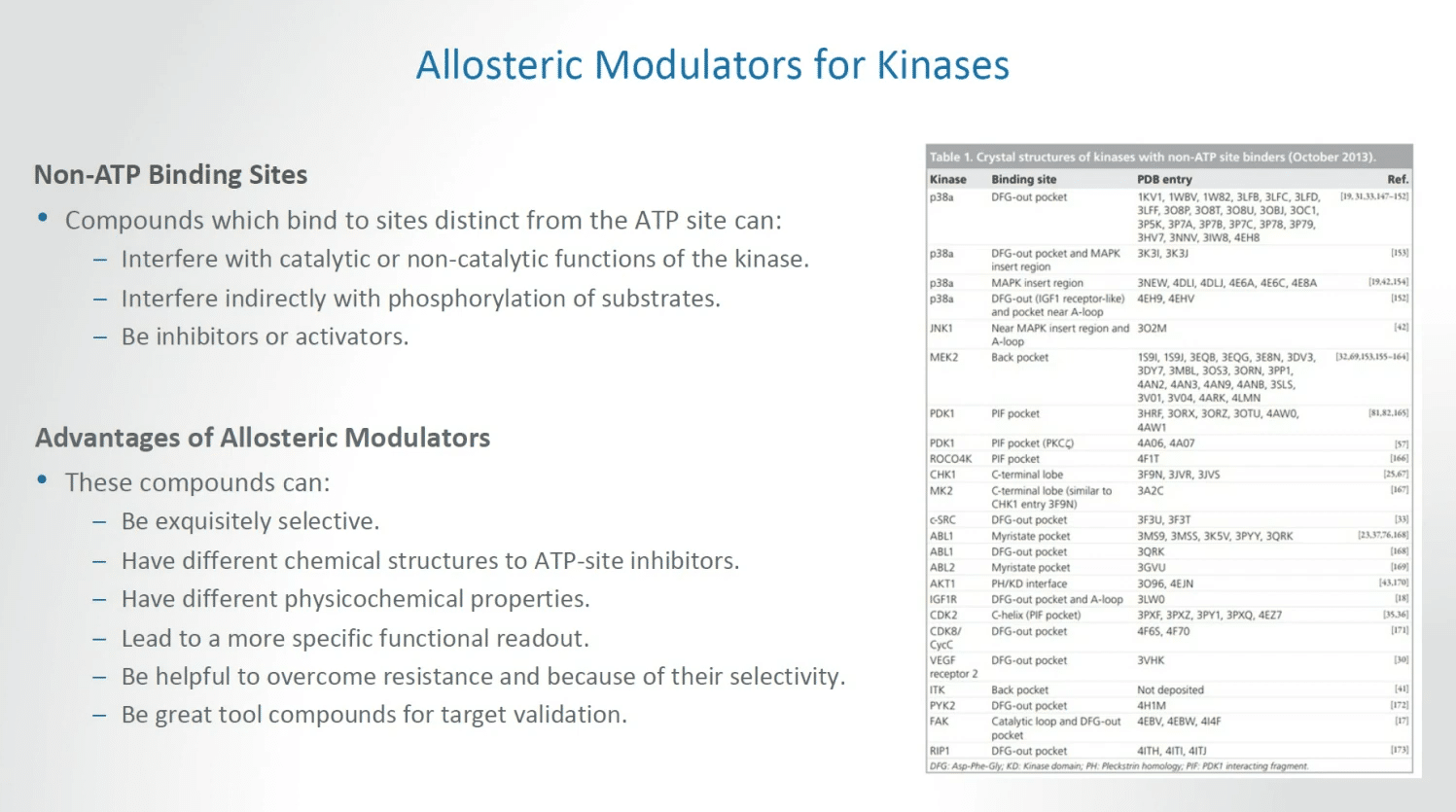
Allosteric modulators, continuing the theme for kinases, there are opportunities in non-ATP binding sites, compounds which bind to sites distinct from the ATP site can interfere with catalytic or non-catalytic functions of the kinase, interfere indirectly with phosphorylation of substrates, and essentially be either inhibitors or activators depending on what is required in a given situation.
Allosteric modulators can lead to compounds that can be exquisitely selective; have different chemical structures to APT site inhibitors; have different physicochemical properties; and can lead to a more specific functional readout; and be helpful to overcome resistance, a key feature of challenges we face, and because of their selectivity become great tool compounds for target validation.

We can also discuss a notion that there is great potential for kinase agonists. Stimulation or enhanced regulation, potentiated regulation of a kinase pathway may be beneficial in some pathologies, and could be essentially achieved by stabilization of the inactive site of a kinase; stabilization of the active state of the kinase; facilitating activation of the kinase. Activators will necessarily be compounds that bind outside the ATP site in our general discussion, and they could lead to the discovery of novel kinase activators which in turn can provide insightful tool compounds and the identification of kinases whose activation can be therapeutic advantages that is hampered currently by the lack of the effective tool compounds that we currently face.
The discovery of novel kinase activators may therefore not only result in drug candidates but could provide products, tool compounds to study the basic biology of kinase activation, leading to ever-increasing research opportunities.
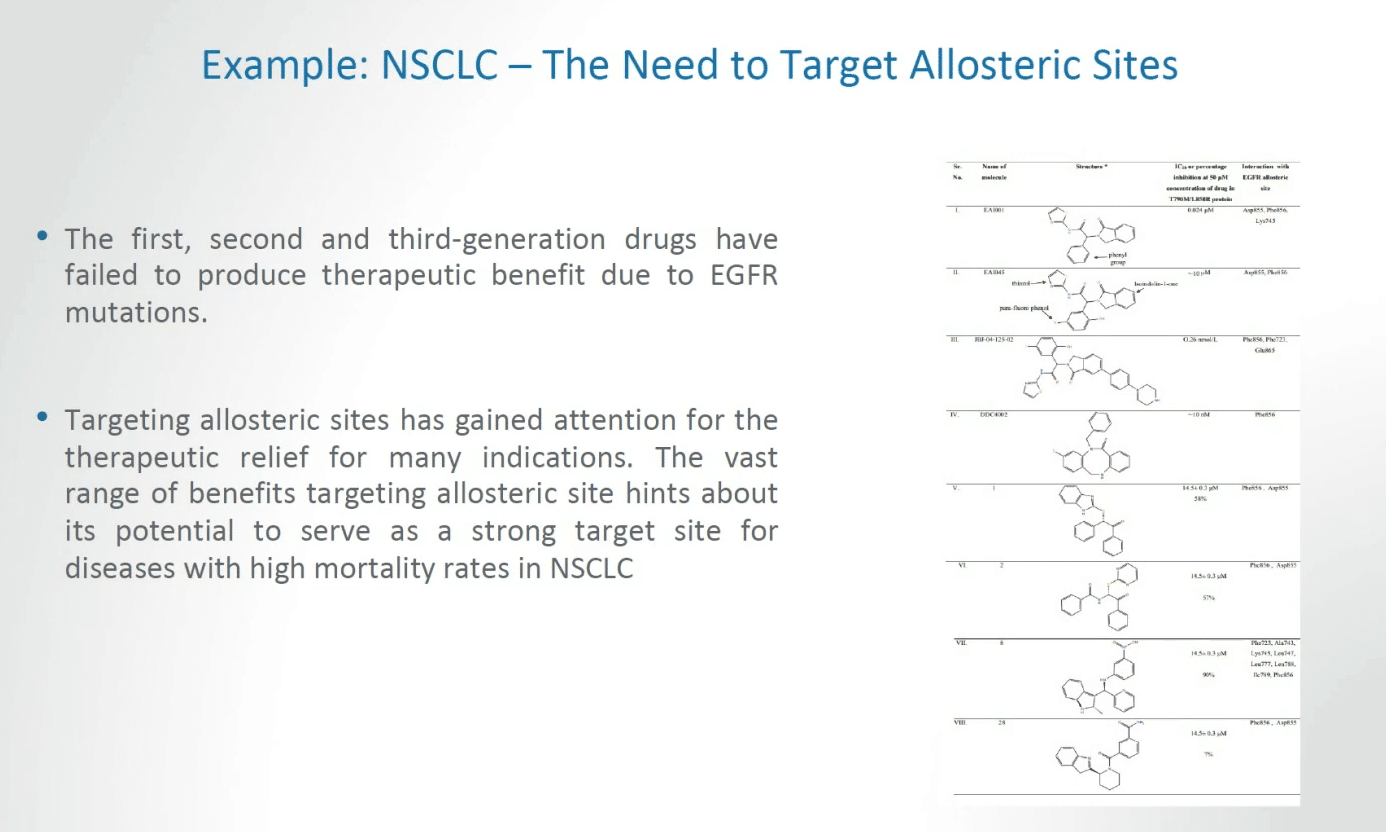
There are certain examples that we already have that give us enormous optimism in understanding where target allosteric discoveries can lead. The first, second and third generation of drugs have in some ways failed to produce therapeutic benefits due to EGFR mutations.
I don’t want to in this context say that we are dealing with total failures. That’s not what I’m saying. But we are dealing with very significant challenges and I think all would agree that we need to approach this challenge in more effective ways.
Targeting allosteric sites has gained attention for the therapeutic relief for many indications. The vast range of benefits targeting allosteric sites essentially hints of the potential to serve as a strong target site for diseases with high mortality rates, for example in non-small cell lung cancer, and these are important considerations in both therapy and advancing the public health.
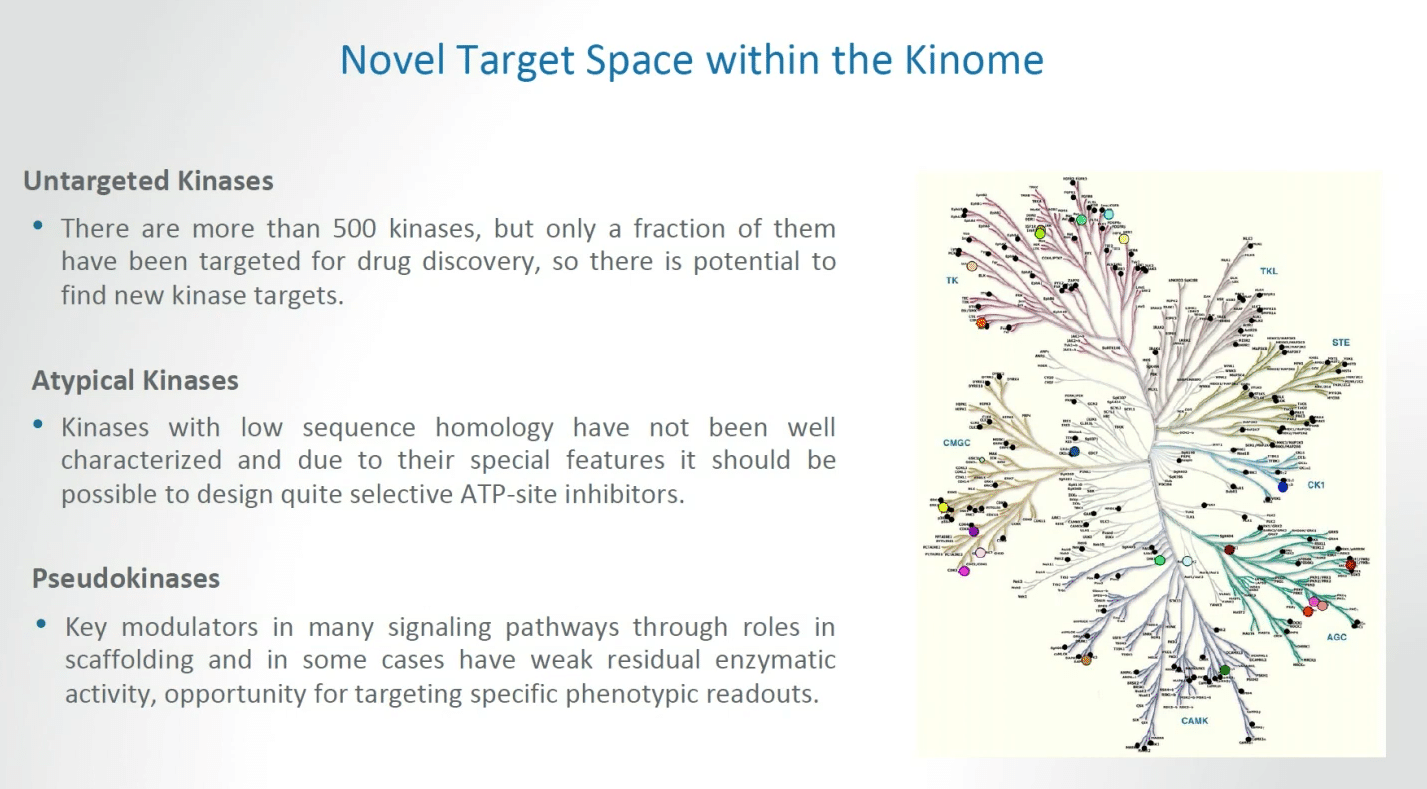
There is novel target opportunities, novel target space within what we call the kinome. This can be broken up into at least three portion. In the setting of untargeted kinases, there are more than 500 kinases but only a fraction of these have been targeted for drug discovery to date, so there is a significant potential to find new kinase targets.
We can also begin the challenge of dealing with atypical kinases, that is kinases with low sequence homology have not been well characterized and due to the special features it should be possible to design selective ATP site inhibitors for this class of kinases.
Finally, there is the whole topic of pseudokinases. That applies to the notion that key modulators in many signaling pathways through roles in scaffolding and in some cases weak residual enzymatic activity can provide opportunities for targeting specific phenotypic readouts.
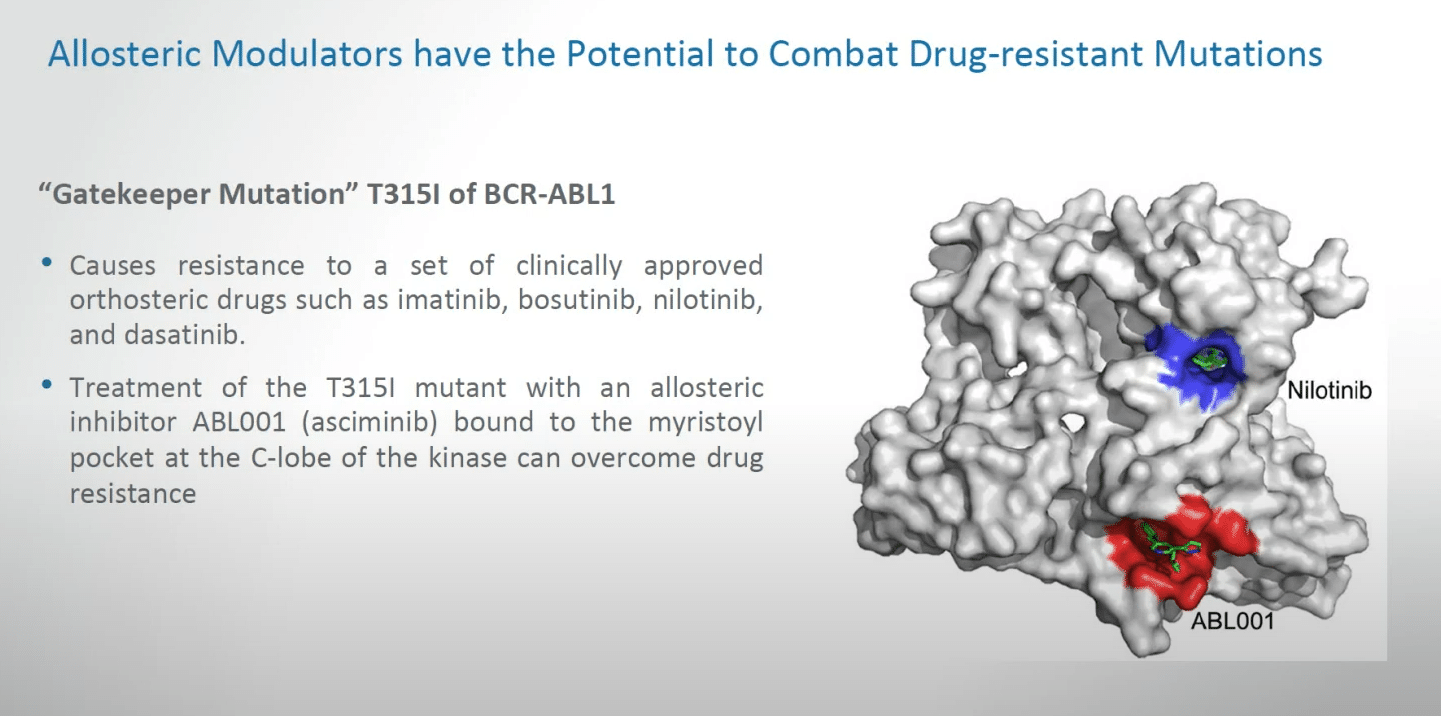
Allosteric modulators have the potential to combat drug-resistant mutations. I’ve approached this several times because as a medical oncologist I cannot list the number of patients whom I have treated personally who despite even early responses have succumbed to a phenomenon of drug resistance, sometimes providing resistance against all known agents. This as a clinician can be a very challenging phase of medical care.
What we can talk about is already some progress, for example, a gatekeeper mutation for the T315 mutation of BCR-ABL. This causes resistance to a set of clinically approved orthosteric drugs that are already on the market, the lead of which was imatinib, and these can provide important opportunities for dealing with resistance, for example in diseases like chronic myelogenous leukemia. The treatment of the relevant mutation with an allosteric inhibitor, which is already available—asciminib—bound to the myristoyl pocket at the C-lobe of the kinase can overcome drug resistance. So, this is a good signal that these things can have practical applications, as shown by the image on the right-hand side.
Next slide.
I want to also give other examples of the potential for allosteric drugs in medical oncology.
So, in effect trametinib, whose trade name is Mekinist, is a highly selective, reversible allosteric inhibitor of MEK1 and MEK2 activity. I want to stress that it is an ATP non-competitive inhibitor that binds MEK adjacent to the ATP binding site. Of very great significance, it has been approved by the FDA for the combination treatment of unresectable metastatic melanoma with BRAF mutations in a very clinical applicable sense.
I think it’s important to remind everybody that once the Food and Drug Administration or EMA or other regulatory bodies approve an agent, that is a remarkable demarcation for both clinical and basic research. Allosteric therapy may have implications in the Src homology-2, so-called SH2 domain, containing protein tyrosine phosphatase 2, the so-called SHP2 target that’s encoded by PTPN11. You’ve already heard a little bit about this already. The latter is a critical allosteric phosphatase for many signaling pathways. SHP2 is a non-receptive protein tyrosine phosphatase encoded by the PTPN11 gene and it is critical in cell growth and differentiated via MAPK signaling pathway. SHP2 also plays an important role in program cell death pathway, effectively what is called a PD-L1 pathway. Mutations in the gene that encodes for SHP2 have been linked to a variety of cancers, while SHP2 also enables activity in the cancer associated Ras-MAP signaling pathway.
I want to emphasize briefly the PD-L1 part of this story, partially because that target is emerging as one of the most important targets, not only in oncology but in all of pharmacology and pharmaceutical development. By the year 2025 is it estimated that Keytruda, which belongs to this class of therapy, will have revenue of $25 billion, with a B, in the year 2025. So we’re talking about major public health and commercial implications linked to some of the processes that we’re talking about today.
Allosterically controlling the ubiquitin-proteasome system has important implications in cancer therapy on a wide scale, and there are enormous strong implications for allosteric interventions targeting tumor growth directly, and targeting the tumor microenvironment, and this is extremely important because we now are realizing that not only do we have to worry about the tumor per se, but the tumor microenvironment, which can have enormous controlling features as to whether a patient will respond or not respond to therapy. Just as an example, the action of the collagen binding receptor tyrosine kinase discoidin, the discoidin domain receptor 2, DDR2, in both tumor and tumor stromal cells has been established as a critical feature for breast cancer metastasis. So these are important features of future research.

I want to end by talking about the future opportunities for Gain Therapeutics. I am extraordinarily enthusiastic about Gain Therapeutics based on my interactions with the brilliant scientific staff at many levels, and I want to just indicate that proteome-scale analysis, which we’ve discussed very briefly, of protein allosteric regulation perturbed by somatic mutations in at least 7,000 cancer genomes have already been reported in the literature, and I expect that list to keep going up.
This provides significant opportunities to utilize the SEE-Tx platform that we’ve heard about and related proprietary technologies at Gain Therapeutics. These technologies at Gain Therapeutics have already identified an allosteric target for our critically important oncogene. And again, I want to emphasize that there’s great enthusiasm not only within the pharmaceutical world, but in the various governmental bodies such as the National Institutes of Health and related agencies, to pursue the goal of diminishing cancer mortality by at least 50%, and again, I emphasize that I think Gain will have an enormous role to play in this particularly important goal.

I want to thank everyone for their attention. Thank you so much.


