Gain Therapeutics targeting allosteric sites: Traditional small molecule drugs work by targeting an active binding site. Gain Therapeutics is focused on targeting allosteric binding sites, a site that is distinct and separate from the traditional binding site. But why is this important? And what role does targeting these sites play in addressing disease?
What are enzymes?
Enzymes are proteins that are found everywhere in nature. They regulate our bodily functions through chemical reactions. Thousands of chemical reactions are controlled by enzymes, and they are often named for their actions. For example, lactase breaks down lactose from dairy products. Lactase breaks down lactose into a form that our intestines can absorb, and when the body has a lactose deficiency, it results in lactose intolerance. Enzymes regulate everything from the formation of our genetic code to our body’s energy production. It is easy to see how enzymes are crucial to support life, but how do they work?
How do enzymes work?
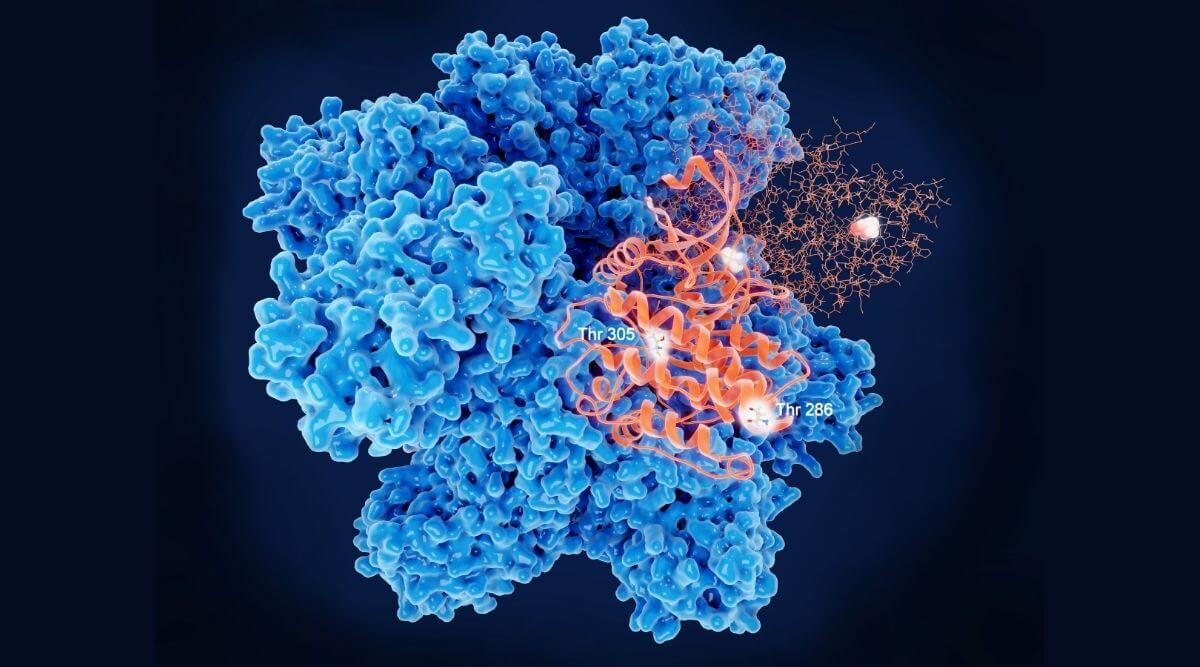
As with all proteins, the sequence of amino acid building blocks determines the final structure of an enzyme and, therefore, the enzyme function. The target of an enzyme is known as the substrate, and the part of the enzyme that interacts with the substrate is the active site. This interaction is like two puzzle pieces with a complimentary fit. The overall folding of the protein maintains the exact orientation of the active site that is necessary for the enzyme to carry out its function. The substrate must bind the active site, like a key in a lock, before any chemical reaction can take place. For some enzymes, this interaction is highly specific like a lock with a single key. However, some enzymes can be promiscuous, acting on many substrates. Once this interaction occurs, the enzyme undergoes a change in its shape and becomes active, allowing it to carry out its function. The substrate binding to the active site begins the chemical reaction, but other factors can adjust this process. One way this can occur is through allosteric binding.
What is allosteric binding sites?
Allosteric binding sites are areas on the enzyme, distinct from the active site, that other molecules can bind. When a biological molecule in the cell binds one of these pockets, the shape of the enzyme can change. This signals a change to the active site which can change the enzyme’s activity, altering the rate of the resulting chemical reaction. This process is known as allostery, or allosteric regulation, which can inhibit or activate an enzyme. By doing this, allosteric binding can speed up or slow down a reaction. Not all enzymes have allosteric binding sites, but enzymes that do are often large molecules with multiple subunits comprising the overall structure. Allosteric regulation occurs all the time within our cells, controlling when, where, and how long chemical reactions take place.
Can we develop drugs to allosteric binding sites?
As noted above, allosteric regulation occurs naturally within our cells, influencing a wide spectrum of chemical reactions when all proteins and enzymes are working properly. However, defective, or misfolded proteins can result from aging, disease, or genetic mutations which ultimately cause abnormal protein function. In both cases, the result is improper function or no function at all. What if the normal shape and function could be restored?
Gain Therapeutics is addressing protein misfolding through use of their SEE-Tx Discovery Platform. This technology platform uses the 3D structure of proteins to identify allosteric binding hotspots on proteins. This enables the supercomputer to screen millions of compounds to discover novel molecules that can target the allosteric sites. The scientists at Gain can then optimize the pool of candidate molecules to find which work best. By doing this, it opens possibilities to regulate proper protein folding within the cell, restoring protein or enzyme function. There are many advantages to the approach of allosteric modulation. The drug would not compete with the natural enzyme substrate compared to drugs that target the enzyme active sites. This means the small molecules would have high specificity for the enzyme but also high efficacy because they would not be competing with another molecule.
Allosteric regulation is a particularly attractive therapeutic strategy for diseases caused by enzyme deficiencies such as Gaucher Disease. In Gaucher disease, a genetic mutation results in insufficient activity of the glucocerebrosidase enzyme in the lysosome, or garbage disposal, of the cell. Glucocerebrosidase breaks down fatty molecules in the cells so when it malfunctions, certain fats can build up to harmful levels. If a small molecule could restore the activity of Glucocerebrosidase, it should restore enzyme function and help modify disease or alleviate the symptoms for patients. Allosteric modulation as a therapeutic strategy has widespread implications for providing new and highly effective treatment options across many classes of disease.