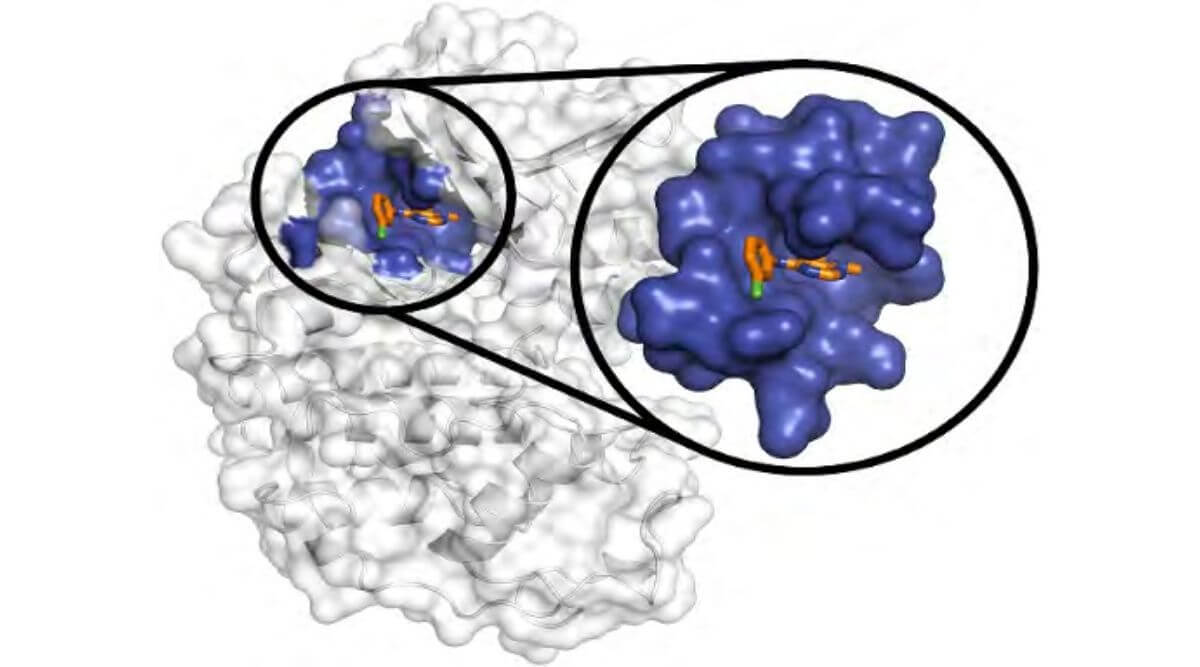
Gain Therapeutics’ SEE-Tx patented algorithm provides quantitative predictions of druggable binding hot spots based on changes in binding free energy (ΔG). No other company has an algorithm like Gain Therapeutics that can quantify druggability of binding sites. This method can discover “hidden” or previously undruggable targets. But with many other drug discovery approaches how does Gain Therapeutics’ SEE-Tx specifically compare?
Gain Therapeutics SEE-Tx Vs. Traditional High-throughput Screening Methods
What is High-throughput screening (HTS)? High-throughput screening (HTS) is a method used in drug discovery to identify drug candidates that can affect a specific target in a desired way. These candidates are referred to as “leads” or “hits”. HTS is used widely in industry as a method of quickly testing the activity of a large quantity of drug-like molecules.
There are however, significant limitations in this approach and with the advent of newer technologies it has become antiquated. When compared to a platform like SEE-Tx which uses molecular dynamics and proprietary druggability analysis to refine the compound pool, HTS requires a physical collection of millions of compounds, robots to handle the experiments, specialised analytic equipment, etc.
Gain Therapeutics’ algorithm is able to narrow down millions of compounds to 100-200 leads. It is much quicker to set up an assay to test 100 compounds than for millions of compounds. The former can be carried out in standard 96-well plates or using biophysical techniques that are not suitable for HTS (e.g. SPR), whereas the latter must be performed in ultra-miniaturised formats that take a lot of time and effort to set up. Typically, we find that 10% of the compounds we select are active. In HTS the hit rate is much lower (<0.1%).
Gain Therapeutics SEE-Tx Vs. Artificial Intelligence based Drug Discovery Platforms
Drug discovery and development is an incredibly time consuming and expensive process–it can take more than 10-15 years for a drug to be approved and costs $2.8B on average. Additionally, only about 14% of drugs make it through all 3 stages of clinical trials and are eventually approved.
In an effort to make this process more efficient both from a time and financial perspective, there have been a lot of companies trying to apply artificial intelligence (AI) to drug development. Because of AI’s ability to analyze large sets of existing data and learn specific patterns and relationships that humans may not be able to recognize due to the subtlety or complexity, the drug discovery space in theory, would be an excellent application.
There are a number of companies with AI driven drug discovery platforms that have produced drug candidates currently in clinical trials. Despite this, AI approaches face significant challenges, especially in regards to data. AI algorithms need experimental data and previous knowledge on the system to train, parameterize and build the predictive models.
On the contrary, SEE-Tx methodology is based on first principles, meaning it doesn’t need any training data or previous knowledge on the system, which makes it applicable to any target with an available 3D structure.
Additionally there is no uncertainty in the datasets with SEE-Tx . The 3D structures have been well documented in literature and the protein’s role in disease has been validated. As data sets improve, AI approaches will no doubt as well-but there will always be some degree of uncertainty in the training site.
SEE-Tx is a solution to the inefficiencies of the drug discovery and development process by greatly reducing the time and cost in discovering novel targets. Currently, there is no known AI algorithm able to induce the opening of hidden or “cryptic” binding sites. This requires a complex and specific coordination of ligand effects on the protein surface which can only be done with SEE-Tx.
Image Source: DOI: 10.1038/NCHEM.2660


4 thoughts on “What makes Gain Therapeutics’ SEE-Tx Platform Unique when compared to Other Drug Discovery Platforms?”
Comments are closed.