Watch the full presentation video of Computational Drug Discovery With Gain Therapeutics Matthias Alder
In conversation with Mathias Alder, CEO of Gain Therapeutics discussing his personal life insights and professional achievements in the field of Computational Drug Discovery.
-
00:00 Matt Pillar:
A few months ago, I set out to learn as much about computational biology and as myriad
applications in Biotech as I could. And on more than one occasion, that effort led me to a Zoom
call with Matthias Alder. That my inquiry resulted in multiple opportunities to talk with
Matthias made me happy. One, because he’s a really great guy, and two, because while his
company’s drug discovery model leans heavily into proprietary structure based computational
methods, he’s not one of these bombastic, over the top futurists who’s all in on relinquishing
science to the algorithms. Just the opposite, he’s pragmatic and practical about the advantages
and the limitations of deploying computational tools in an industry whose mission it is to save
lives by injecting things into living human bodies.
I’m Matt Pillar. This is the business of biotech. And on today’s show, I’m bringing you a conversation with my friend Matthias Alder, newly appointed CEO at Gain Therapeutics. Matthias, welcome to the show. - 01:10 Matthias Alder: Thanks for having me, Matt. A real pleasure to be here and look forward to the conversation.
- 01:15 Matt Pillar: It’s a pleasure to have you. It’s great that, as I said, we’ve talked on the phone and on Zoom here a few times, and I’ve enjoyed every minute of it. I really enjoy your conversation with you and your perspectives on this topic, and I’m glad we could finally get you on the podcast. Before we get into some of the particulars around what Gain is doing with computational, though, I want our audience to have a chance to get to know you a little bit better. So, I want to rewind back to your academic days. You’ve got a couple of law degrees. One, I know you got a law degree from Miami, I believe, and there was one before that, right?
- 01:54 Matthias Alder: Right
- 01:56 Matt Pillar Straight away went to work in a large law firm, and at some point, your interests either changed to or zeroed in on life sciences. So take us back to that i just want to kind of get a feel for how things started for you coming out of school with a law degree and then working your way into the life sciences.
- 02:15 Matthias Alder Absolutely and I’m originally from Switzerland. My first law degree was actually from the University of Boston in Switzerland, and so I went to the US, got some international exposure, came back to Switzerland and wanted to work in the international environment, having some using what I’ve learned over that second during the course of that second degree. And in Switzerland you can go either into insurance, banking or pharmaceuticals, and so that’s the choices that you have, and more by chance than by choice, I ended up actually working at one of the large pharma companies in Switzerland as my first job in the legal field. And immediately I felt that thrill of working on something that’s bigger than just creating the next widget right, or moving money around. What we’re doing in our industry is really benefiting all of humanity in the end. And being part of that process, even as a lawyer at the time, I thought was really cool. And so, that got me started. I then moved over to the US with my wife at the time, still is my wife, actually, who I got to know when I was in the US for my postgraduate law degree. So, she came to Switzerland. We moved back together in the mid-nineties, and I joined that law firm by name of Cooley, which was specializing in representing biotech companies. And that was a very conscious choice I made. I wanted to stay in the field and work with biotech companies, help them be successful. And so, I developed a very extensive sort of deal sheet, transactional sheet, lots of MNA, lots of licensing deals, collaboration deals that ultimately then led me to want to join the business as posted just being an advisor, and then jumped into my first company as a TC at the time, 2006, I think it was.
- 04:25 Matt Pillar Yeah. So back in school, there wasn’t necessarily like a focus or a concentration in life sciences that kind of came after you left, after you graduated with it.
- 04:40 Matthias Alder Yeah, it came after, and in a way, much to my surprise, because, so the reason I went into law school was because my strengths weren’t math or physics or biology, and so I had to choose a different path. But I always had an interest, and what I really felt interested in is the science transactions and deal making. That was sort of how I came into being where I am today, and being able to take complex concepts and explain them in a way in layman’s terms, which is actually helpful if you’re not a scientist, you actually have to think through things in that much sort of simpler ways and more structurally. And that was really helpful. I don’t get lost in the weeds, but I can sort of step up and look at the big picture.
- 05:36 Matt Pillar Yeah, I was going to say, I’ve had enough conversation with you to know that you’re probably not giving yourself enough credit for your ability to express the technical and scientific jobs because you do quite a good job at it.
- 05:48 Matthias Alder I appreciate that.
- 05:49 Matt Pillar You did establish, just through General Counsel Work and Consultative Work in the Legal Counsel Space, what looked like a promising career there. At what point and why did you decide to sort of transition from being zeroed in on the legal aspects to, I think, sort of the Chief Business Officer and then on up through the Trajectory to your current role as CEO? What was the catalyst for that transition?
-
06:26 Matthias Alder
Yeah, I wouldn’t call it a midlife crisis, really, but I was sort of in the middle of my career and looking at what’s going to do. I did really well. I was a partner at the firm. I had a great client base with lots of interesting deals that I was doing. But in the end, it was essentially doing the same thing over and over again, always the same thing. There was not really a progression from in terms of the professional experience I could have because in all humbleness, I was really good at what I was doing. So, I negotiated deals. It counts them all together. It’s in excess of $10 billion worth of BioBox that I’ve accumulated in terms of deals and transactions I’ve worked on. But they were always the same in the end you knew exactly, you know, I’m going to say this, and then the other side is going to say that, and then we come back with this. And you sort of just know how the game was played. And I looked at them and thought, I could do that for the next 20 years. Or maybe I should look at exploring different horizons and then it’s very logical move then to look at the clients that I had and thought, which of the clients would I want to join and have to join one of my clients as their first in house general counsel. And from there on, it was sort of a natural progression as I got more involved in the business on the business side, business development side, not just doing the deal negotiation at the end, but building up all of the interactions leading up to a deal ultimately. And also on the operational side, I became responsible in a couple of the companies I’ve been at for Human resources and build organizations from small sort of 30 to 50 people organizations to 200, 400 sized people sized organizations. A lot of operational experience of what it takes to build a company and along the way picking up all the challenges that we are facing in drug development, the things to anticipate the hurdles that we face. And so, that over time then led me to now where I am today at Gain, really integrating that entirety of my professional experience as the CEO at Gain and it’s been a recent advancement to that role, but it’s been incredibly thrilling and rewarding so far. - 08:50 Matt Pillar Yeah, and I want to ask you a little bit about your objectives as far as the new role as CEO again is concerned. But before I do, I’m curious when you paint that picture about your transition from the focus on the legal aspects to Chief Business Officer and the Chief Operations Officer and I’m the CEO suite position, you sort of frame that up like you know like you wanted to expand your influence and your opportunity to be influential whether to a company beyond legal aspect. I’m curious about your reflections on your findings when you made those moves. Was the grass truly greener on the other side? Were their aspects you know those transitions that you feel, particularly inadequate to unprepared for perhaps or so what were your findings along the way, I guess?
- 9:50 Matthias Alder Every transition that I have made has been, you know I wasn’t prepared for what I was gonna take on. Every step of the way in terms of my entire career but I was always focused on taking what I had already known what I have learned what I know how to do build nothing expanding to something else never set to do something more that’s related but building on its that you can see you know for being an lawyer at a big Swiss pharma company while you’re really small car get there in the system to becoming something legal lawyer for Biotech companies as an outside adviser to become the GC for a company, first I was a deal guy then I had beef up on all the to see all things she was a publicly traded company so really getting up the curve on that and from there on then expect sorry expanding onto into business development and the interactions looking back and also looking forward it felt like a natural progression at each step of what I was going to do next. I have always been interested in science, technology and people. And, at every step of the way it was adding further elements in different directions in terms of my experience.
- 11:08 Matt Pillar Yeah, very good so, when I first met you few months back you see you were not CEO at Gain. You were Chief Operation Officer Maybe.
- 11:16 Matthias Alder Yes, exactly.
- 11:18 Matt Pillar So, the CEO role it’s a gone weeks or couple of months ago may be.
- 11:23 Matthias Alder Couple of months ago.
- 11:25 Matt Pillar Mid-September Yeah, so with that new title and the handing of the rings that worrier. I, guess early kind of objectives that were sort of in front of you.
-
11:42 Matthias Alder
I think I was actually very happy to with the way how the transition happened. The plan was always the former CEO engagement time already has looked for the success I had joined. So, the anticipation of me eventually taking over that I joined Gain and it played out couldn’t have played out any better really for so I had 40 years’ worth of time to get fully engrained in the company understanding you know the platform that we talk about in just a minute understanding the program the science behind it, getting to know the people and so forth and so it’s been a very seamless transition and so it’s not I’m not in a situation where I’m coming as a CEO and so what’s actually going so I know, what’s actually going on we’re in terms of from me taking over easily just pushing forward down the path that we have already set ourselves on and that is focused really on three areas one is very firmly focused on executing on the lead program that we have in Parkinson’s disease that program is moving through preclinical stage late preclinical development is getting into the clinic in the middle of next year. So very much an execution task they are making sure that things stay on track and we have the project team working together and anticipating all of the things that need to be done on a towards getting the company to for the first time by becoming a clinical stage company that’s going to be a major transition for us.
The other area of focus which is also one of the reasons why the company the board was interested in in bringing me in is business development, they have a very extensive business development background and we have numerous opportunities as a company with our assets that we have on the both pipeline side. But also, with the platform to engage in very interesting transactions collaborations and licensing deals with pharma company’s and other industry in the company’s and so there’s a big focus on that for me to move that forward and getting deals done over the coming period. - 14:09 Matt Pillar Yeah
- 14:10 Matthias Alder And then finally yeah there’s an obviously in the current market environment there’s a lot of engagement that with investors that need needs to take place because we need to tell the story be out there, keep up the awareness because there’s a lot of competition for attention and we want to be one of the companies that the people think of when they think about investing in the sector. The computational drug discovery sector or those companies with neurodegenerative disease programs.
- 14:41 Matt Pillar Yeah, yes and on the former of those two points uh the cop you know computational biology uh company sector um it’s an increasingly busy space right we see a lot of a lot of companies making claims anyway to be leveraging computational biology in their drug discovery effort several you know tons of companies that are creating platforms that you know perhaps not with zero intention of creating their own pipeline or building their from candidates they’re looking to be service providers solely it’s just a very very very busy space right now I mean I’ve got my theories on why that is you know you mentioned the restricted capital markets there’s a lot of expectation that by pharmaceutical companies especially new emerging ones do more quickly with fewer resources and I believe this is a technology could step into to fill some of those gaps but again um you know you’re your roots there are deep you’re a company who is not I wouldn’t classify you as one of these maybe academically rooted companies who is sort of having to take a step backwards and revisit where computational might fit within its structure Gain you know the computational approaches is seeded very deeply right and again so I want to learn a little bit about that tell us you know before we get into the platform itself.
-
16:05 Matthias Alder
Right, - 16:06 Matt Pillar Theoretically fundamentally why is computational so central to Gain’s approach.
- 16:14 Matthias Alder Well, I think it goes back to the founding of the company which was it’s actually founded based on the platform that was developed, you mentioned we’re not through scientifically that typically found that we have actually had the personnel who has developed the platform is our chief technology officer Sharrei Parrel. He was and still is a professor at the university of Barcelona so and he has had the opportunity to uh create this platform and then the Gain was founded about five years ago around the platform.
- 16:48 Matt Pillar Hum
- 16:50 Matthias Alder And the interesting part of the platform is it really has come it has developed over time and has come to fruition really only in the last 18 months or so because of like we are now able to combine the advances in in computational power through supercomputing that becomes increasingly accessible now with the Artificial Intelligence powered advances in structural biology. These are all big words but we can ask we’re going to go through the platform I can it’s a bit more specific and I think it touched on an important point there’s a lot of companies out there that have very flashy websites and things moving around but ultimately all comes down to that you know the technology and the science behind it and I think what differentiates us from many of those companies is that we have actually applied our platform to build the entirety of our pipeline that we have identified about nine programs with the platform with the lead program moving into clinical development in the middle of this coming year. so it’s a very well validated platform or it’s not just a hypothetical construct that we have created but we have tangible evidence it actually works great valuable drug development program for us.
- 18:13 Matt Pillar When it was I hypothetical construct right like when it was an idea and perhaps this predates humanities and that that’s fine I just want to get your perspective, what you know about the company what it was a theoretical idea or hypothetical.
- 18:30 Matthias Alder Right
- 18:31 Matt Pillar What drove the what if like I said like right now, I believe there’s a market opportunity for technologies like this because the what if is what if we could extend our cash runway by doing things a lot more efficiently and then in less time on the discovery side that’s the driver right in in my mind that’s ever least
- 18:50 Matthias Alder Right
- 18:51 Matt Pillar You know back then what was the what it was it was it more like you know technology is cool, big data is cool we’re not leveraging it enough we could probably do something cool here or was it you know we want to build something that we can sell what sort of that motivated.
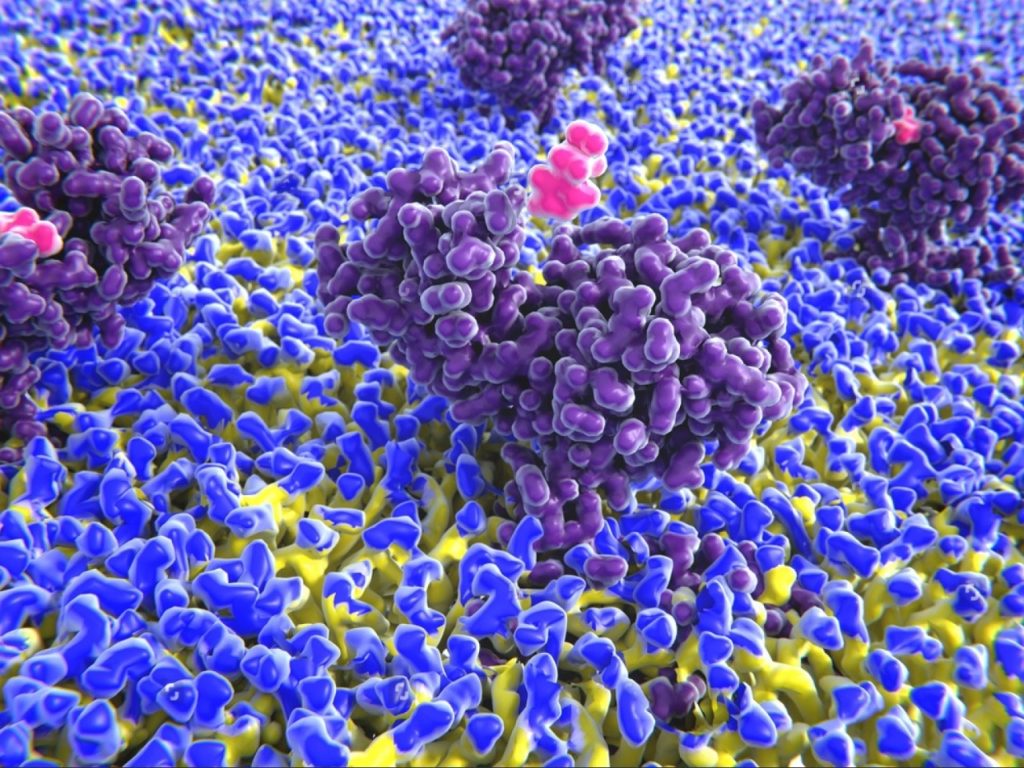
- 19:05 Matthias Alder It was actually much more fundamental and driven by in a way a shortcoming of what drug development look like at the time, in that you know many of our human diseases are caused by proteins either doing too much or too little and don’t function correctly but only about 10% of those proteins are druggable because they have a known binding site. Where you can engage with a product to actually interact with the protein to impact it in the way that’s desired to affect the disease. 90% of proteins have been at the time undruggable until then what if was the question was that job he asked himself is what it if we could develop a platform a technology that allows us to target those 90% of proteins that are not druggable because they don’t have an old binding site. So the focus was on developing a platform and the technology that allows us to identify new binding sites new ways new places where products can interact with the protein surface that never been seen before and even on proteins that have previously been considered not druggable because there was no way to find, you know these places of interaction and so that was the origin and so he developed the platform Gain eventually got created based on that platform and with the platform we have now shown in multiple programs that we can identify noble binding sites on proteins that have you know previously been undruggable, we have also been able to show that with and that’s the additional benefits that that we have with the platform, is that we can accelerate the drug discovery process from what typically with traditional methods take two plus years through high though screening. So, in physics based says if you’re if you’re doing that with the computational platform, we can accelerate that process two to less than three months. So an incredible gain in time that we can achieve and if you’re thinking about the drug development process, you know drug discovery dropped it in preclinical development clinical development that takes up to 10 years and we’re cutting off the initial two years of drug discovery and reducing that to three months it’s just incredibly powerful tool and as you said pharma companies increasingly are looking at how can we be more efficient, how can we speed up the development process because time is money and every you know months spent in discovery that is that is not is not really advancing the program and so that’s why I think we are at the really good stopped and at the sweet spot really in terms of where the industry is moving and where we can come in with our platform to feel a need.
- 22:09 Matt Pillar Yeah, ok now I’m going to give you the moment you’ve been I’m waiting for where we can actually talk about the platform I’ll stop with all my you know rambling preamble questions but it’s important question because you know without a discussion around what you’re doing in the platform how it works what’s going in what’s coming out now you know, a lot of this machine learning computational biology how do you talk and get chucked up to you know some like black box, you know voodoo magic right understanding around you know what the application looks like so. So, let’s start with data. I’ve had plenty of conversations with people in this space, who talk about the struggle the data struggle right like there’s a there’s a lot of big they call big data for reason there’s a lot of data out there and attempts to leverage like publicly available data or unstructured data they’re from the cloud and myriad other resources. Right, have been met with challenge you know because it’s unstructured because it’s you know because there are anomalies in the data yeah, in your case the data that’s gone that’s feeding the machine is proprietary is that correct. So, let’s start with like what’s going in and in the first place.
- 23:32 Matthias Alder Absolutely, so particularly fell. I don’t know inadequately you’re right then there’s a lot of talk about big data and if you know especially companies in the artificial intelligence field are and machine learning field our reliance on a lot of data to teach their computational models what to look for and to learn Where we are very different from those companies is that we don’t actually need any big data at all, what we are what the platform is it’s a structure based and a physics based platform and the only data that we need is information that we need it is the structure of the protein that we are that we want to investigate with the platform and the proteins structure originally used to you know you have to get to through computer through experimental methods by your yen X-ray crystallography and NMR other tools to create that or define what the structure is of the protein and we can use that for the platform that’s really helpful but what literally has it exploded the target universe for us is the is the arrival of alpha fold, which is a an AI powered platform that Google has developed for their deep mind subsidiary company and with that platform what we can predict the structure the 3D structure all the protein from just the one the oligonucleotide sequence and so with that access to alpha fold which is an open source platform, they’re using AI in a way but it’s not core to our platform it’s a it gives us the starting point of what we need which is the protein structure.
- 25:23 Matt Pillar Yeah,
- 25:24 Matthias Alder Once we have the protein structure that we build that into the platform there’s a lot of it takes sort of one to two weeks for us to look at the structure analyse it building into the platform and what we doing with the structure in the in the platform is interrogating the entire surface of the protein with small molecule probes with organic molecules to measure computationally the binding interaction seeing where these small molecules go how they interact with the protein surface we measure these interactions.
- 26:02 Matt Pillar Yeah,
- 26:03 Matthias Alder Then we analyse these that the binding free energy down the is interactions and they’re able to categorize identify binding hotspots, so where do most of the molecules go, we have multiple probes that we’re using and we’re do many of them go what kind of uh where is that spot are there other hot other binding around that and to that hotspot sit in a structural fold that were a product as small molecule action that onto the surface of the protein and so we’re able to use that platform takes one to two weeks to come up with that binding spot. Where we can find there will be suitable for intervention through with are molecules we are working with. That’s the first step.
- 26:53 Matt Pillar I’m going to back up really quick with that quick follow up question on step one and early step one what sort of guide rails or I guess what guides the decision around the determination of which proteins are you going to interrogate.
- 27:12 Matthias Alder I think that is very much driven by the disease that we’re looking at and so that’s a company we’re not doing strictly speaking target discovery so we’re not trying to find new proteins and figure out what these proteins do in a particular disease so we are relying on literature to review saying, ok this is a protein that plays a role, if it doesn’t work then then a disease develops and so we’re taking that information as a basis to select our targets for our drug discovery programs. So, it’s really based on or if the other people have done but it really gives us a leg up in terms of starting at a point that’s already validated.
- 28:00 Matt Pillar Yeah, ok thanks for clearing that up it’s an import because you mentioned from the outset, you’re like you know we don’t necessarily need big data but you know if you if you if your organ proteins that we already know are associated with a specific indication you know you could get to a big real big data situation right really quick.
- 28:22 Matthias Alder Yeah right
- 28:23 Matt Pillar So you just took us through step one sort of how the analysis and assessment takes place, like what does that analysis or assessment look like, when it’s potentially success we’re going to take actually successful because you know we’re going to get to this part of the conversation you don’t run the spit out a win, there’s more to it beyond that but it you run the algorithm and you spit out to win a perhaps a better indication that you are close right!
- 28:55 Matthias Alder Right yeah precisely, so the what the initial step in the platform where we’re identifying a binding site does is running at a molecular dynamic model it gives us literally hundreds of potential binding sites on a protein and what we do then is apply a proprietary patented algorithm to analyze specifically these binding sites and in the end after some screening campaign on a protein we end up with two maybe three potential binding sites that we believe have real value and you and a lot of that is not just looking at numbers and you know it’s not like an app that spits out a result a lot of that is also used in the experience of the of the computational team and net cam team that we have at the company working with this platform for many years to understand the data and what it really means in numbers and understand what the binding part would have to look like to be to be a promising binding pocket and all that so there’s production of human assessment and analysis that needs to go into finding the appropriate binding sites, we end up with two to virtual screening campaign, where we’re looking at knowing at an atomic level. What the binding hotspots are within a binding pocket that we have found. We’re looking for molecules that fit onto these binding hotspots and fit structurally into the pocket the size of the pocket that we have.
- 30:22 Matt Pillar Hmm
- 30:23 Matthias Alder And we’re running a docking protocol there’s other people who do that as well those as you mentioned there is there are competitors out there who use some of the similar methods but we have a differentiator again with our methodology that we’re not only looking at how our molecule binds onto the surface we also look at how much power it takes to pull it off so an undocking protocol that we run and that allows us to eliminate it 80 to 90% of molecules that appear to be good candidates for drug development but then turn out not too bad to be binding tightly enough and so again it’s an important step and again that’s a proprietary step that we applied that we then allows us to pick off the probably thousand 2000 or so that we that we get from a virtual screening campaign we picked the 100 or so molecules that are the most promising different chemistries to really then to the that’s the third step then do the in labs or real life vet lab, assessment of what these what these compounds actually do in real life experiments and I think there is overloaded to that you know it’s it is it all AI is it all computational it is it’s actually not but we believe what we do is we get to a starting point very quickly and terms of knowing that the structure and the molecules but then we always need to confirm, whether the predictions that the model is making we need to confirm that prediction in real experiments in real life to know whether or not what we have style is the winner or not.
- 32:08 Matt Pillar Yeah there’s a couple a couple of folks I want to ask you know in in that vein right your easier your transition from INSELECO will go to you know to vet lab I often hear that the term Unicorn used uh when folks try to describe the scientists, who is well you know adapt at both computational biology and you know traditional research science but right like this person doesn’t exist and I and I think you know I think that’s an overstatement I think it’s changing I think academia is probably paying a little bit more attention through that now and putting out some you know some fresh young talent that’s a little bit more savvy on both sides of that spectrum but that being said I you know I’ve had conversations with biotech leaders such as you know folks in in similar roles to you uh who talk about the need to jump suppose somehow or entrench, the IT side of the talent pool and the you know more traditional biology side of the of the talent pool how do you do that at Gain so that’s so that’s the first follow up to what you just said like that that transition I mean how do you make sure that the right hand on the left hand are holding hands so to speak.
- 33:32 Matthias Alder Yeah, I think it’s pretty organic really within the company because that’s how the company was set up and it’s really just another you know element or evidence or instance of interest interdisciplinary science right and you can’t and I think that’s right the key here need to have people are open to looking at the science in India in different ways and being open to pick putting what initially appear to be different pieces really putting together it aren’t to a whole and we have done that really successfully in gain that we have of our computational team that focuses primarily on looking on finding the binding sites and the virtual hits they’re sitting right next to our medicinal chemists, who then look at the straw factors that come out that the virtual screening and identify red flags or green flags always to rescreen things in a in a better way and they sit right next to uh the our people who are in in their labs and you know when they write reports they sit in the same office as the people in the actual vet lab experiments in the in the labs that we have in that same location and so it’s just a very natural and integrated way of how we are operating, which is why we also think truly, it is a platform even though they are different pieces that we are putting pieces together where we are it can only work in the it only works because everybody is working together in an integrated fashion.
- 35:08 Matt Pillar Yeah, the other follow-up I had uh was around I I guess the acknowledgement of the scepticism around machine learning you were you were careful to say like you know we don’t rely entirely on the output of the algorithm we then you know take great care to take it to the world lab and can confirm order you know confirm or reject right I’m fine.
- 35:35 Matthias Alder Precisely.
- 35:36 Matt Pillar So I’m curious about your you know even beyond just what’s going on in Gain what your worldview is on you know or your vision for responsibly ensuring that what’s happening in these INSELECO activities, you know activities is now and will continue to into the future translate into in view you know safety and efficacy alright like just sort of like, I said big picture how do we everybody fears right like I mean you know you watch some sci-fi movies you come away thinking the algorithm are we’re going to take over the world talk about these very real as I said in the in the intro we’re talking about relying to some degree on machines and algorithms to help us develop products that will make their way into the human body hopefully to therapeutic effect. How do we make sure that we’re making that transition responsibly.
- 36:31 Matthias Alder Well I think that this system is how I saw big picture wise I think this is how we’re using technology in the best way it’s real to aid and speed up but not replace you know the human factor in terms of what it what is required to do developers are a safe and effective drug, I mentioned right so they drop the process of getting a drug to market you have to discover we faced, the preclinical development phase and the clinical development phase and what we’re essentially doing with our platform is taking care of the initial drug discovery phase so we’re getting through that very quickly you know less than three months compared to two plus years and then the work really starts in terms of making sure the molecule that we have identified the drug is actually effective and actually safe and so we are from that point on going through the traditional process of drug development. So we’re doing the in vitro models the in vivo models we’re going to preclinical talks and then we’re taking it into the Phase-1 study and so forth and so we’re not actually trying to short circuit and take the molecule straight from in vitro into a human, where I think that the real there would be a real concern about what are you guys doing but it’s really speeding up the initial phase and which is already a huge value that we can contribute in this in this overall process.
- 38:04 Matt Pillar Yeah, ok so one of the big questions I have for you is like to what and are you experiencing success as game with its with its therapeutic pipeline and you mentioned I think a little bit ago you mentioned that you got eight or nine eight or nine candidates that have yeah have been you know born out of at least some element of your application of your own platform technology. So, tell us a little bit about that just give us some colour on I guess examples of where contributions from the plate form have led to or lent to uh the clinical progress of your own internal problem.
- 38:45 Matthias Alder Right, well I think if you’re looking at our pipeline there is a significant block of that is in lysosomal storage disorder and going back to that point about how we pick our protein these are all caused by these lysosomal storage disorders are caused by genetic mutations that impact an enzyme that plays a critical role in cell health and
- 39:10 Matt Pillar Hmm
- 39:11 Matthias Alder So these enzymes you know couldn’t be targeted before right you did you didn’t really what you need to do is like Quite novel which is what we’ve been able to do so we found on these enzymes a place for a where a compound can interact with the surface to restore proper folding and restore the function of the enzyme and that’s a very unique approach that has really been enabled by our platform that allowed us to specifically look for products that actually have that effect that would have been otherwise possible and as a result we have been able to develop, this it’s very robust applied pipeline license storage disorders. In terms of selection of the ends and or actually, the can’t take the credit for that it was actually the our scientific team was really smart about that in that they picked or the program that we have in Gaucher disease, they picked the enzyme that is also responsible for about 14% of Parkinson’s patients too you have to start to develop Parkinson’s disease so it’s the same you patient uh from that same gene that expresses that same enzyme that is misfolded that plays a role both in Gaucher and in Parkinson’s disease and now based on that has allowed it to expand our rare disease is uh a program into the much larger indication of Parkinson’s disease and that’s the one that we’re now taking into the clinic and so from you know it it’s been really rewarding to see that the thought that went into that applying the platform and now being able to reap the fruits of all that early smart thinking that the company has been able to do.
- 41:01 Matt Pillar Yeah, and as far as taking that Parkinson’s candidate into the clinics concerned, I’ll also note for the benefit of our audience that work is caught some you know the attention of some important players in that space and very influential players in that spaceship collaborating with the Michael J fox foundation, on that project which is good company to have when you’re when you’re moving into a space like that.
- 41:26 Matthias Alder It is uh really rewarding to have gains that support from the Michael J fox foundation and they’ve been a big supporter initially with a financial support to get the program progressing to where we are today and at this point we continue to work very closely with them on you know framing out new programs, looking out the clinical program looking at biomarkers that are important in Parkinson’s disease that we can measure looking at disease progression disease symptoms and all of that it’s a very it’s an ongoing very close collaboration that we have with the foundation we’re very grateful for their support.
- 42:09 Matt Pillar Hmm, what is the next step in terms of the clinical activity before the Parkinson’s candidate.
- 42:17 Matthias Alder So, with that program we are now in in late three clinical development we’re going through with the toxicology studies animals studies that are required before we are allowed to take that products into humans that work is progressing while we’re on track to us start the uh to make the direct required filings for start the phase one study in the middle of the year and then take it into the clinic from there on so it’s well in hand and well progressing well.
- 42:48 Matt Pillar Good and as you as you plan that clinical activity you know you just mentioned part of your work with Michael J fox foundation question is around discovery of biomarkers and other things that indicate to me that perhaps there was an application there’s an application potential for computational approach approaches machine learning or might be on the clinical side as well you know that’s efficiency.
- 43:10 Matthias Alder Yes
- 43:11 Matt Pillar Patient population identification you know plenty of outpatients there probably and you and I spoke briefly about this not too long ago, so I want to give you an opportunity to talk about what you envision or intend to do with these high-tech approaches
- 43:28 Matthias Alder Right
- 43:31 Matt Pillar As you move from you know development into clinical.
- 43:34 Matthias Alder And that’s a thank you for raising that’s a very important aspect obviously being a company rooted in on the computational side with our drug discovery platform, we are acutely aware and interested in in exploiting the these advances that really help accelerate or streamline or special make increase the likelihood of success of a clinical program and so we’re looking at a number of platforms that other companies have developed many of them using AI, big data to really source out some of these disease aspects and that allows us maybe potentially to select, have a better patient selection in our clinical studies to ensure that we have the appropriate patients for the product that we are developing. The other aspects include looking at some more hardware like tools that I can’t play a real role in terms of wearable’s looking at symptom progression or lack thereof in the clinical study in Parkinson’s in particular, there’s now you know wristbands and things like that before ongoing measurements in terms of the locomotion impairment, the gait and things like that that we can apply in our clinical study to capture that data and make sure that we’re making the right choices as were developed in developing the product in these patients.
- 45:02 Matt Pillar Yeah, very cool very exciting of an opportunity again an opportunity for game to be on and uh the leading some of the applications for that new technology very good, all right we’re uh we’re kind of running short on time here, uh with this but I got I Public relations people holding back but when I reflect on what Gain is today what it’s come from and what it’s become today. I see this company that’s built up form that in and of itself is a is a valuable, you know product right to the life sciences community to the to the pharmaceutical biopharmaceutical community and a lot of companies that are trying to do that you know check that CTX platform that you’ve developed and you can just say that’s what we do you know we offer that as a service even to you know to other pharmaceutical companies but I also see a company that’s developed a pipeline and is making you know significant preclinical progress as planned is it going to the clinic next year and I know that there are multiple answers to this question but what’s the end game for you know Matthias’s vision for gain therapeutics do you want to you know do you want to be a service provider slash therapeutic developer do you want to be a you know three to early be clinical developer and then you know sell off assets do you want kind of develop stuff that goes all the way to the commercial finish line like what sort for the we always talk about and you know begin with the end in in mind is there an end in mind for Gain.
- 46:40 Matthias Alder Some tongue in cheek I would say. You said it to all of the above and that’s the way the way I think about Gain is truly we have two asset pockets that we can leverage one is the pipeline that we have generated with the platform and there we have a lead program in Parkinson’s he’s moving into the clinic we have polo on programs and that I expose the opportunity for intrinsic value creation by the developing these products and creating more evidence that they will actually work and creating more investor confidence. They also provide an opportunity for partnering so as a small company it’s very clear that we are not never ever going to be able to develop all nine programs in parallel and take them all the way to market and so we are very active in looking at engaging with potential partners in the Pharmaceutical to take licenses, engage in collaborations with us which can help us generate no that would give cash inflow for us from licensed revenues and things like that so we’re looking at the pipeline from both angle and the same really for the platform. The platform allows us both to generate additional programs early on that that are actually it’s incredibly inexpensive for us to develop the its initial programs because the computational methods are already there we need just need to rent some supercomputing time and run them and we can come up with new programs in a split second almost, but we’re also looking at the platform from the bank in terms of this it should be valuable and is valuable to you know the Pharmaceutical industry to potential partners who may want to engage into in discovery collaborations with us if they have a protein target of interest that they haven’t been able to find anything to work with we can actually take that protein on our platform and spit out that the other end a product that then that the partner can take on take back and develop themselves and so again there it’s in inherent value creation as well as well as value creation through collaborations and we’re pursuing you know all of these aspects in parallel so for my perspective I’m over incredibly well positioned with the company with gain, to take this take us forward and through this very turbulent time that we now see that capital markets and that has an impact all across the industry but with the assets that that that we have the opportunities of value creation that we have I’m very optimistic about what we can achieve with our team here at Gain in terms of progressing company to which to the next level.
- 49:31 Matt Pillar Yeah, well-done response to that question because it was thorough yet vague open-ended enough off to keep on PR and IR police have pretty, so thank you whatever I asked you Mattie is that uh is central to the story what are what you know what should I have asked you that I did not ask you.
- 49:55 Matthias Alder Well I to me we’re uh in the biotech industry and I mentioned that uh before at the reason we’re here and the reason we’re doing what we’re doing is to develop new therapies for patient think patients in need and we haven’t really spent a lot of time talking about that or Parkinson’s program but we are really excited about what we have there because with our approach we are essentially um correcting an enzyme that is the protein in the body that’s not working right and because we are correcting that enzyme right to restore the healthy cell so it’s a very sort of uh fundamental approach that we are pursuing so we’re creating with our with our product we’re creating a healthy cell which then that cell provides longer and that happens specifically also with dopaminergic neurons so these are cells that produce dopamine and the lack of dopamine in the brain ultimately causes I have Parkinson’s symptoms and so with our approach were able to right store the function of the enzyme and the and every store that healthy selling store every store the survival of the cell and as a result restored the production of dopamine in the brain and with that we have the ability to have a disease modify in Parkinson’s patients that currently only get their symptoms treated and eventually be they get worse and worse and worse overtime yeah and so having the opportunity here with getting to be at the forefront in a very severe and significant disease in for doing so significant more for this day needs that’s what it that’s what it’s all about and that has us you know getting up every morning and going to work for patients to provide that benefit um and look forward to that
- 51:56 Matt Pillar Yeah
- 51:57 Matthias Alder The coming years to achieve to achieve that big goal.
- 52:01 Matt Pillar Yeah, definitely and you know I guess it’s important to point you probably already did but just to sort of reinforce force that act that activity that happens at the enzymatic level is post like it’s done without any genetic modification it’s after the genes and events work so I think I think I mean you know I’m not necessarily and observe you know I talk to a lot of folks but it occurs to me that the potential safety advantages and also complexity the advantages over you know perhaps some of the genetic therapies true you know true gene therapies other that are looking at this indication. Yeah, it occurs to me that there are probably some advantages on the Gain’s approach.
- 52:46 Matthias Alder There are and there is it’s obviously with the only ones developing uh you know new blacks in Parkinson’s disease gene therapy is out there are a number of challenges with these approaches and you know there’s a number of high profile, you know failure shall we say in terms of gene therapy programs. The other aspect is that gene therapy ultimately is always going to be a very expensive therapy and especially for larger indications like Parkinson’s disease, it’s not quite demanded imaginable how any healthcare system can actually really afford paying that therapy for patients who need it and so having an approach like we have which is a small molecule approach is standard way to develop its standard way to manufacture it standard way to commercialize it we’re not trying to forge a new path here it’s going to come out with an effect that is can be gene therapy like in that we’re fixing the enzyme that that gets misfolded because of a genetic mutation so we’re fixing the enzyme and have the opportunity to have that disease modifying effect that you in therapy also has the potential to have and so it’s a great opportunity I think we’re competitive really well positioned we’re clearly the leading program with our mechanism of action and it’s our job to push it through and make it make it that the you know.
- 54:20 Matt Pillar Yeah,
- 54:23 Matthias Alder Fulfil the promise that we currently holding our hands.
- 54:28 Matt Pillar Sure, yeah you your job now but you got the you took the CEO role to come down and
- 54:33 Matthias Alder Happy to take it on and there and bring it forward bridge absolutely
- 54:38 Matt Pillar Well, that’s excellent Matthias I really enjoy talking with you, I appreciate you coming on to through the story with us it’s a fascinating work. I think there’s a super insightful for our audience I love these application stories around computational I appreciate the detail you gave us so thank you for joining us and
- 54:55 Matthias Alder uh you know thank you Matt I’ll make sure we stay in touch and we’ll do it again sometime wonderful much appreciated thanks for the opportunity great to talk to you.
- 55:04 Matt Pillar Yeah you too so .That’s game therapeutic CEO Matthias Alder I’m Matt and this is the business of biotech, in case you missed it we just launch the newsletter to accompany this program and I’d like you to sign up for it at bio processonline.com\ Bob I’d also like you to show sativa some love for its support of this project by going to cytiva.com\ emerging biotech to check out all the great resources they’ve procured for new emerging biotech in the meantime be sure to subscribe and leave us to review.


In conversation with Mathias Alder, CEO of Gain Therapeutics discussing his personal life insights and professional achievements in the field of Computational Drug Discovery.
Matt Pillar: A few months ago, I set out to learn as much about computational biology and as myriad applications in Biotech as I could. And on more than one occasion, that effort led me to a Zoom call with Matthias Alder. That my inquiry resulted in multiple opportunities to talk with Matthias made me happy. One, because he’s a really great guy, and two, because while his company’s drug discovery model leans heavily into proprietary structure based computational methods, he’s not one of these bombastic, over the top futurists who’s all in on relinquishing science to the algorithms. Just the opposite, he’s pragmatic and practical about the advantages and the limitations of deploying computational tools in an industry whose mission it is to save lives by injecting things into living human bodies.
I’m Matt Pillar. This is the business of biotech. And on today’s show, I’m bringing you a conversation with my friend Matthias Alder, newly appointed CEO at Gain Therapeutics. Matthias, welcome to the show.
Matthias Alder: Thanks for having me, Matt. A real pleasure to be here and look forward to the conversation.
Matt Pillar: It’s a pleasure to have you. It’s great that, as I said, we’ve talked on the phone and on Zoom here a few times, and I’ve enjoyed every minute of it. I really enjoy your conversation with you and your perspectives on this topic, and I’m glad we could finally get you on the podcast. Before we get into some of the particulars around what Gain is doing with computational, though, I want our audience to have a chance to get to know you a little bit better. So, I want to rewind back to your academic days. You’ve got a couple of law degrees. One, I know you got a law degree from Miami, I believe, and there was one before that, right?
Matthias Alder: Right
Matt Pillar: Straight away went to work in a large law firm, and at some point, your interests either changed to or zeroed in on life sciences. So take us back to that i just want to kind of get a feel for how things started for you coming out of school with a law degree and then working your way into the life sciences.
Matthias Alder: Absolutely and I’m originally from Switzerland. My first law degree was actually from the University of Boston in Switzerland, and so I went to the US, got some international exposure, came back to Switzerland and wanted to work in the international environment, having some using what I’ve learned over that second during the course of that second degree. And in Switzerland you can go either into insurance, banking or pharmaceuticals, and so that’s the choices that you have, and more by chance than by choice, I ended up actually working at one of the large pharma companies in Switzerland as my first job in the legal field. And immediately I felt that thrill of working on something that’s bigger than just creating the next widget right, or moving money around. What we’re doing in our industry is really benefiting all of humanity in the end. And being part of that process, even as a lawyer at the time, I thought was really cool. And so, that got me started. I then moved over to the US with my wife at the time, still is my wife, actually, who I got to know when I was in the US for my postgraduate law degree. So, she came to Switzerland. We moved back together in the mid-nineties, and I joined that law firm by name of Cooley, which was specializing in representing biotech companies. And that was a very conscious choice I made. I wanted to stay in the field and work with biotech companies, help them be successful. And so, I developed a very extensive sort of deal sheet, transactional sheet, lots of MNA, lots of licensing deals, collaboration deals that ultimately then led me to want to join the business as posted just being an advisor, and then jumped into my first company as a TC at the time, 2006, I think it was.
Matt Pillar: Yeah. So back in school, there wasn’t necessarily like a focus or a concentration in life sciences that kind of came after you left, after you graduated with it.
Matthias Alder: Yeah, it came after, and in a way, much to my surprise, because, so the reason I went into law school was because my strengths weren’t math or physics or biology, and so I had to choose a different path. But I always had an interest, and what I really felt interested in is the science transactions and deal making. That was sort of how I came into being where I am today, and being able to take complex concepts and explain them in a way in layman’s terms, which is actually helpful if you’re not a scientist, you actually have to think through things in that much sort of simpler ways and more structurally. And that was really helpful. I don’t get lost in the weeds, but I can sort of step up and look at the big picture.
Matt Pillar: Yeah, I was going to say, I’ve had enough conversation with you to know that you’re probably not giving yourself enough credit for your ability to express the technical and scientific jobs because you do quite a good job at it.
Matthias Alder: I appreciate that.
Matt Pillar: You did establish, just through General Counsel Work and Consultative Work in the Legal Counsel Space, what looked like a promising career there. At what point and why did you decide to sort of transition from being zeroed in on the legal aspects to, I think, sort of the Chief Business Officer and then on up through the Trajectory to your current role as CEO? What was the catalyst for that transition?
Matthias Alder: Yeah, I wouldn’t call it a midlife crisis, really, but I was sort of in the middle of my career and looking at what’s going to do. I did really well. I was a partner at the firm. I had a great client base with lots of interesting deals that I was doing. But in the end, it was essentially doing the same thing over and over again, always the same thing. There was not really a progression from in terms of the professional experience I could have because in all humbleness, I was really good at what I was doing. So, I negotiated deals. It counts them all together. It’s in excess of $10 billion worth of BioBox that I’ve accumulated in terms of deals and transactions I’ve worked on. But they were always the same in the end you knew exactly, you know, I’m going to say this, and then the other side is going to say that, and then we come back with this. And you sort of just know how the game was played. And I looked at them and thought, I could do that for the next 20 years. Or maybe I should look at exploring different horizons and then it’s very logical move then to look at the clients that I had and thought, which of the clients would I want to join and have to join one of my clients as their first in house general counsel. And from there on, it was sort of a natural progression as I got more involved in the business on the business side, business development side, not just doing the deal negotiation at the end, but building up all of the interactions leading up to a deal ultimately. And also on the operational side, I became responsible in a couple of the companies I’ve been at for Human resources and build organizations from small sort of 30 to 50 people organizations to 200, 400 sized people sized organizations. A lot of operational experience of what it takes to build a company and along the way picking up all the challenges that we are facing in drug development, the things to anticipate the hurdles that we face. And so, that over time then led me to now where I am today at Gain, really integrating that entirety of my professional experience as the CEO at Gain and it’s been a recent advancement to that role, but it’s been incredibly thrilling and rewarding so far.
Matt Pillar: Yeah, and I want to ask you a little bit about your objectives as far as the new role as CEO again is concerned. But before I do, I’m curious when you paint that picture about your transition from the focus on the legal aspects to Chief Business Officer and the Chief Operations Officer and I’m the CEO suite position, you sort of frame that up like you know like you wanted to expand your influence and your opportunity to be influential whether to a company beyond legal aspect. I’m curious about your reflections on your findings when you made those moves. Was the grass truly greener on the other side? Were their aspects you know those transitions that you feel, particularly inadequate to unprepared for perhaps or so what were your findings along the way, I guess?

Matthias Alder: Every transition that I have made has been, you know I wasn’t prepared for what I was gonna take on. Every step of the way in terms of my entire career but I was always focused on taking what I had already known what I have learned what I know how to do build nothing expanding to something else never set to do something more that’s related but building on its that you can see you know for being an lawyer at a big Swiss pharma company while you’re really small car get there in the system to becoming something legal lawyer for Biotech companies as an outside adviser to become the GC for a company, first I was a deal guy then I had beef up on all the to see all things she was a publicly traded company so really getting up the curve on that and from there on then expect sorry expanding onto into business development and the interactions looking back and also looking forward it felt like a natural progression at each step of what I was going to do next. I have always been interested in science, technology and people. And, at every step of the way it was adding further elements in different directions in terms of my experience.
Matt Pillar: Yeah, very good so, when I first met you few months back you see you were not CEO at Gain. You were Chief Operation Officer Maybe.
Matthias Alder: Yes, exactly.
Matt Pillar: So, the CEO role it’s a gone weeks or couple of months ago may be.
Matthias Alder: Couple of months ago.
Matt Pillar: Mid-September Yeah, so with that new title and the handing of the rings that worrier. I, guess early kind of objectives that were sort of in front of you.

Matthias Alder: I think I was actually very happy to with the way how the transition happened. The plan was always the former CEO engagement time already has looked for the success I had joined. So, the anticipation of me eventually taking over that I joined Gain and it played out couldn’t have played out any better really for so I had 40 years’ worth of time to get fully engrained in the company understanding you know the platform that we talk about in just a minute understanding the program the science behind it, getting to know the people and so forth and so it’s been a very seamless transition and so it’s not I’m not in a situation where I’m coming as a CEO and so what’s actually going so I know, what’s actually going on we’re in terms of from me taking over easily just pushing forward down the path that we have already set ourselves on and that is focused really on three areas one is very firmly focused on executing on the lead program that we have in Parkinson’s disease that program is moving through preclinical stage late preclinical development is getting into the clinic in the middle of next year. So very much an execution task they are making sure that things stay on track and we have the project team working together and anticipating all of the things that need to be done on a towards getting the company to for the first time by becoming a clinical stage company that’s going to be a major transition for us.
The other area of focus which is also one of the reasons why the company the board was interested in in bringing me in is business development, they have a very extensive business development background and we have numerous opportunities as a company with our assets that we have on the both pipeline side. But also, with the platform to engage in very interesting transactions collaborations and licensing deals with pharma company’s and other industry in the company’s and so there’s a big focus on that for me to move that forward and getting deals done over the coming period.
Matt Pillar: Yeah
Matthias Alder: And then finally yeah there’s an obviously in the current market environment there’s a lot of engagement that with investors that need needs to take place because we need to tell the story be out there, keep up the awareness because there’s a lot of competition for attention and we want to be one of the companies that the people think of when they think about investing in the sector. The computational drug discovery sector or those companies with neurodegenerative disease programs.

Matt Pillar: Yeah, yes and on the former of those two points uh the cop you know computational biology uh company sector um it’s an increasingly busy space right we see a lot of a lot of companies making claims anyway to be leveraging computational biology in their drug discovery effort several you know tons of companies that are creating platforms that you know perhaps not with zero intention of creating their own pipeline or building their from candidates they’re looking to be service providers solely it’s just a very very very busy space right now I mean I’ve got my theories on why that is you know you mentioned the restricted capital markets there’s a lot of expectation that by pharmaceutical companies especially new emerging ones do more quickly with fewer resources and I believe this is a technology could step into to fill some of those gaps but again um you know you’re your roots there are deep you’re a company who is not I wouldn’t classify you as one of these maybe academically rooted companies who is sort of having to take a step backwards and revisit where computational might fit within its structure Gain you know the computational approaches is seeded very deeply right and again so I want to learn a little bit about that tell us you know before we get into the platform itself.
Matthias Alder: Right,
Matt Pillar: Theoretically fundamentally why is computational so central to Gain’s approach.
Matthias Alder: Well, I think it goes back to the founding of the company which was it’s actually founded based on the platform that was developed, you mentioned we’re not through scientifically that typically found that we have actually had the personnel who has developed the platform is our chief technology officer Sharrei Parrel. He was and still is a professor at the university of Barcelona so and he has had the opportunity to uh create this platform and then the Gain was founded about five years ago around the platform.
Matt Pillar: Hum
Matthias Alder: And the interesting part of the platform is it really has come it has developed over time and has come to fruition really only in the last 18 months or so because of like we are now able to combine the advances in in computational power through supercomputing that becomes increasingly accessible now with the Artificial Intelligence powered advances in structural biology. These are all big words but we can ask we’re going to go through the platform I can it’s a bit more specific and I think it touched on an important point there’s a lot of companies out there that have very flashy websites and things moving around but ultimately all comes down to that you know the technology and the science behind it and I think what differentiates us from many of those companies is that we have actually applied our platform to build the entirety of our pipeline that we have identified about nine programs with the platform with the lead program moving into clinical development in the middle of this coming year. so it’s a very well validated platform or it’s not just a hypothetical construct that we have created but we have tangible evidence it actually works great valuable drug development program for us.

Matt Pillar: When it was I hypothetical construct right like when it was an idea and perhaps this predates humanities and that that’s fine I just want to get your perspective, what you know about the company what it was a theoretical idea or hypothetical.
Matthias Alder: Right
Matt Pillar: What drove the what if like I said like right now, I believe there’s a market opportunity for technologies like this because the what if is what if we could extend our cash runway by doing things a lot more efficiently and then in less time on the discovery side that’s the driver right in in my mind that’s ever least
Matthias Alder: Right
Matt Pillar: You know back then what was the what it was it was it more like you know technology is cool, big data is cool we’re not leveraging it enough we could probably do something cool here or was it you know we want to build something that we can sell what sort of that motivated.
Matthias Alder: It was actually much more fundamental and driven by in a way a shortcoming of what drug development look like at the time, in that you know many of our human diseases are caused by proteins either doing too much or too little and don’t function correctly but only about 10% of those proteins are druggable because they have a known binding site. Where you can engage with a product to actually interact with the protein to impact it in the way that’s desired to affect the disease. 90% of proteins have been at the time undruggable until then what if was the question was that job he asked himself is what it if we could develop a platform a technology that allows us to target those 90% of proteins that are not druggable because they don’t have an old binding site. So the focus was on developing a platform and the technology that allows us to identify new binding sites new ways new places where products can interact with the protein surface that never been seen before and even on proteins that have previously been considered not druggable because there was no way to find, you know these places of interaction and so that was the origin and so he developed the platform Gain eventually got created based on that platform and with the platform we have now shown in multiple programs that we can identify noble binding sites on proteins that have you know previously been undruggable, we have also been able to show that with and that’s the additional benefits that that we have with the platform, is that we can accelerate the drug discovery process from what typically with traditional methods take two plus years through high though screening. So, in physics based says if you’re if you’re doing that with the computational platform, we can accelerate that process two to less than three months. So an incredible gain in time that we can achieve and if you’re thinking about the drug development process, you know drug discovery dropped it in preclinical development clinical development that takes up to 10 years and we’re cutting off the initial two years of drug discovery and reducing that to three months it’s just incredibly powerful tool and as you said pharma companies increasingly are looking at how can we be more efficient, how can we speed up the development process because time is money and every you know months spent in discovery that is that is not is not really advancing the program and so that’s why I think we are at the really good stopped and at the sweet spot really in terms of where the industry is moving and where we can come in with our platform to feel a need.
Matt Pillar: Yeah, ok now I’m going to give you the moment you’ve been I’m waiting for where we can actually talk about the platform I’ll stop with all my you know rambling preamble questions but it’s important question because you know without a discussion around what you’re doing in the platform how it works what’s going in what’s coming out now you know, a lot of this machine learning computational biology how do you talk and get chucked up to you know some like black box, you know voodoo magic right understanding around you know what the application looks like so. So, let’s start with data. I’ve had plenty of conversations with people in this space, who talk about the struggle the data struggle right like there’s a there’s a lot of big they call big data for reason there’s a lot of data out there and attempts to leverage like publicly available data or unstructured data they’re from the cloud and myriad other resources. Right, have been met with challenge you know because it’s unstructured because it’s you know because there are anomalies in the data yeah, in your case the data that’s gone that’s feeding the machine is proprietary is that correct. So, let’s start with like what’s going in and in the first place.
Matthias Alder: Absolutely, so particularly fell. I don’t know inadequately you’re right then there’s a lot of talk about big data and if you know especially companies in the artificial intelligence field are and machine learning field our reliance on a lot of data to teach their computational models what to look for and to learn Where we are very different from those companies is that we don’t actually need any big data at all, what we are what the platform is it’s a structure based and a physics based platform and the only data that we need is information that we need it is the structure of the protein that we are that we want to investigate with the platform and the proteins structure originally used to you know you have to get to through computer through experimental methods by your yen X-ray crystallography and NMR other tools to create that or define what the structure is of the protein and we can use that for the platform that’s really helpful but what literally has it exploded the target universe for us is the is the arrival of alpha fold, which is a an AI powered platform that Google has developed for their deep mind subsidiary company and with that platform what we can predict the structure the 3D structure all the protein from just the one the oligonucleotide sequence and so with that access to alpha fold which is an open source platform, they’re using AI in a way but it’s not core to our platform it’s a it gives us the starting point of what we need which is the protein structure.
Matt Pillar: Yeah,
Matthias Alder : Once we have the protein structure that we build that into the platform there’s a lot of it takes sort of one to two weeks for us to look at the structure analyse it building into the platform and what we doing with the structure in the in the platform is interrogating the entire surface of the protein with small molecule probes with organic molecules to measure computationally the binding interaction seeing where these small molecules go how they interact with the protein surface we measure these interactions.
Matt Pillar: Yeah,
Matthias Alder: Then we analyse these that the binding free energy down the is interactions and they’re able to categorize identify binding hotspots, so where do most of the molecules go, we have multiple probes that we’re using and we’re do many of them go what kind of uh where is that spot are there other hot other binding around that and to that hotspot sit in a structural fold that were a product as small molecule action that onto the surface of the protein and so we’re able to use that platform takes one to two weeks to come up with that binding spot. Where we can find there will be suitable for intervention through with are molecules we are working with. That’s the first step.
Matt Pillar: I’m going to back up really quick with that quick follow up question on step one and early step one what sort of guide rails or I guess what guides the decision around the determination of which proteins are you going to interrogate.
Matthias Alder: I think that is very much driven by the disease that we’re looking at and so that’s a company we’re not doing strictly speaking target discovery so we’re not trying to find new proteins and figure out what these proteins do in a particular disease so we are relying on literature to review saying, ok this is a protein that plays a role, if it doesn’t work then then a disease develops and so we’re taking that information as a basis to select our targets for our drug discovery programs. So, it’s really based on or if the other people have done but it really gives us a leg up in terms of starting at a point that’s already validated.
Matt Pillar: Yeah, ok thanks for clearing that up it’s an import because you mentioned from the outset, you’re like you know we don’t necessarily need big data but you know if you if you if your organ proteins that we already know are associated with a specific indication you know you could get to a big real big data situation right really quick.
Matthias Alder: Yeah right

When you’re striving to excel in a new arena, the best guides are the ones already doing it well. The business of biotech brings those voices forward to help new and emerging biopharma’s turn they’re innovations like MRNA and cell and gene therapies into clinical realities.tune in and subscribe for insights on hiring regulatory and other need to know topics for biopharma leaders. The podcast is brought to you in collaboration with cytiva. check out their resources at sitediva.com\ emerge biotech that’s CYTIV a.com\ emerging biotech .
Matt Pillar: So you just took us through step one sort of how the analysis and assessment takes place, like what does that analysis or assessment look like, when it’s potentially success we’re going to take actually successful because you know we’re going to get to this part of the conversation you don’t run the spit out a win, there’s more to it beyond that but it you run the algorithm and you spit out to win a perhaps a better indication that you are close right!
Matthias Alder: Right yeah precisely, so the what the initial step in the platform where we’re identifying a binding site does is running at a molecular dynamic model it gives us literally hundreds of potential binding sites on a protein and what we do then is apply a proprietary patented algorithm to analyze specifically these binding sites and in the end after some screening campaign on a protein we end up with two maybe three potential binding sites that we believe have real value and you and a lot of that is not just looking at numbers and you know it’s not like an app that spits out a result a lot of that is also used in the experience of the of the computational team and net cam team that we have at the company working with this platform for many years to understand the data and what it really means in numbers and understand what the binding part would have to look like to be to be a promising binding pocket and all that so there’s production of human assessment and analysis that needs to go into finding the appropriate binding sites, we end up with two to virtual screening campaign, where we’re looking at knowing at an atomic level. What the binding hotspots are within a binding pocket that we have found. We’re looking for molecules that fit onto these binding hotspots and fit structurally into the pocket the size of the pocket that we have.
Matt Pillar: Hmm

Matthias Alder: And we’re running a docking protocol there’s other people who do that as well those as you mentioned there is there are competitors out there who use some of the similar methods but we have a differentiator again with our methodology that we’re not only looking at how our molecule binds onto the surface we also look at how much power it takes to pull it off so an undocking protocol that we run and that allows us to eliminate it 80 to 90% of molecules that appear to be good candidates for drug development but then turn out not too bad to be binding tightly enough and so again it’s an important step and again that’s a proprietary step that we applied that we then allows us to pick off the probably thousand 2000 or so that we that we get from a virtual screening campaign we picked the 100 or so molecules that are the most promising different chemistries to really then to the that’s the third step then do the in labs or real life vet lab, assessment of what these what these compounds actually do in real life experiments and I think there is overloaded to that you know it’s it is it all AI is it all computational it is it’s actually not but we believe what we do is we get to a starting point very quickly and terms of knowing that the structure and the molecules but then we always need to confirm, whether the predictions that the model is making we need to confirm that prediction in real experiments in real life to know whether or not what we have style is the winner or not.
Matt Pillar: Yeah there’s a couple a couple of folks I want to ask you know in in that vein right your easier your transition from INSELECO will go to you know to vet lab I often hear that the term Unicorn used uh when folks try to describe the scientists, who is well you know adapt at both computational biology and you know traditional research science but right like this person doesn’t exist and I and I think you know I think that’s an overstatement I think it’s changing I think academia is probably paying a little bit more attention through that now and putting out some you know some fresh young talent that’s a little bit more savvy on both sides of that spectrum but that being said I you know I’ve had conversations with biotech leaders such as you know folks in in similar roles to you uh who talk about the need to jump suppose somehow or entrench, the IT side of the talent pool and the you know more traditional biology side of the of the talent pool how do you do that at Gain so that’s so that’s the first follow up to what you just said like that that transition I mean how do you make sure that the right hand on the left hand are holding hands so to speak.
Matthias Alder: Yeah, I think it’s pretty organic really within the company because that’s how the company was set up and it’s really just another you know element or evidence or instance of interest interdisciplinary science right and you can’t and I think that’s right the key here need to have people are open to looking at the science in India in different ways and being open to pick putting what initially appear to be different pieces really putting together it aren’t to a whole and we have done that really successfully in gain that we have of our computational team that focuses primarily on looking on finding the binding sites and the virtual hits they’re sitting right next to our medicinal chemists, who then look at the straw factors that come out that the virtual screening and identify red flags or green flags always to rescreen things in a in a better way and they sit right next to uh the our people who are in in their labs and you know when they write reports they sit in the same office as the people in the actual vet lab experiments in the in the labs that we have in that same location and so it’s just a very natural and integrated way of how we are operating, which is why we also think truly, it is a platform even though they are different pieces that we are putting pieces together where we are it can only work in the it only works because everybody is working together in an integrated fashion.
Matt Pillar: Yeah, the other follow-up I had uh was around I I guess the acknowledgement of the scepticism around machine learning you were you were careful to say like you know we don’t rely entirely on the output of the algorithm we then you know take great care to take it to the world lab and can confirm order you know confirm or reject right I’m fine.
Matthias Alder: Precisely.
Matt Pillar: So I’m curious about your you know even beyond just what’s going on in Gain what your worldview is on you know or your vision for responsibly ensuring that what’s happening in these INSELECO activities, you know activities is now and will continue to into the future translate into in view you know safety and efficacy alright like just sort of like, I said big picture how do we everybody fears right like I mean you know you watch some sci-fi movies you come away thinking the algorithm are we’re going to take over the world talk about these very real as I said in the in the intro we’re talking about relying to some degree on machines and algorithms to help us develop products that will make their way into the human body hopefully to therapeutic effect. How do we make sure that we’re making that transition responsibly.
Matthias Alder: Well I think that this system is how I saw big picture wise I think this is how we’re using technology in the best way it’s real to aid and speed up but not replace you know the human factor in terms of what it what is required to do developers are a safe and effective drug, I mentioned right so they drop the process of getting a drug to market you have to discover we faced, the preclinical development phase and the clinical development phase and what we’re essentially doing with our platform is taking care of the initial drug discovery phase so we’re getting through that very quickly you know less than three months compared to two plus years and then the work really starts in terms of making sure the molecule that we have identified the drug is actually effective and actually safe and so we are from that point on going through the traditional process of drug development. So we’re doing the in vitro models the in vivo models we’re going to preclinical talks and then we’re taking it into the Phase-1 study and so forth and so we’re not actually trying to short circuit and take the molecule straight from in vitro into a human, where I think that the real there would be a real concern about what are you guys doing but it’s really speeding up the initial phase and which is already a huge value that we can contribute in this in this overall process.
Matt Pillar: Yeah, ok so one of the big questions I have for you is like to what and are you experiencing success as game with its with its therapeutic pipeline and you mentioned I think a little bit ago you mentioned that you got eight or nine eight or nine candidates that have yeah have been you know born out of at least some element of your application of your own platform technology. So, tell us a little bit about that just give us some colour on I guess examples of where contributions from the plate form have led to or lent to uh the clinical progress of your own internal problem.
Matthias Alder: Right, well I think if you’re looking at our pipeline there is a significant block of that is in lysosomal storage disorder and going back to that point about how we pick our protein these are all caused by these lysosomal storage disorders are caused by genetic mutations that impact an enzyme that plays a critical role in cell health and
Matt Pillar: Hmm
Matthias Alder: So these enzymes you know couldn’t be targeted before right you did you didn’t really what you need to do is like Quite novel which is what we’ve been able to do so we found on these enzymes a place for a where a compound can interact with the surface to restore proper folding and restore the function of the enzyme and that’s a very unique approach that has really been enabled by our platform that allowed us to specifically look for products that actually have that effect that would have been otherwise possible and as a result we have been able to develop, this it’s very robust applied pipeline license storage disorders. In terms of selection of the ends and or actually, the can’t take the credit for that it was actually the our scientific team was really smart about that in that they picked or the program that we have in Gaucher disease, they picked the enzyme that is also responsible for about 14% of Parkinson’s patients too you have to start to develop Parkinson’s disease so it’s the same you patient uh from that same gene that expresses that same enzyme that is misfolded that plays a role both in Gaucher and in Parkinson’s disease and now based on that has allowed it to expand our rare disease is uh a program into the much larger indication of Parkinson’s disease and that’s the one that we’re now taking into the clinic and so from you know it it’s been really rewarding to see that the thought that went into that applying the platform and now being able to reap the fruits of all that early smart thinking that the company has been able to do.
Matt Pillar: Yeah, and as far as taking that Parkinson’s candidate into the clinics concerned, I’ll also note for the benefit of our audience that work is caught some you know the attention of some important players in that space and very influential players in that spaceship collaborating with the Michael J fox foundation, on that project which is good company to have when you’re when you’re moving into a space like that.
Matthias Alder: It is uh really rewarding to have gains that support from the Michael J fox foundation and they’ve been a big supporter initially with a financial support to get the program progressing to where we are today and at this point we continue to work very closely with them on you know framing out new programs, looking out the clinical program looking at biomarkers that are important in Parkinson’s disease that we can measure looking at disease progression disease symptoms and all of that it’s a very it’s an ongoing very close collaboration that we have with the foundation we’re very grateful for their support.
Matt Pillar: Hmm, what is the next step in terms of the clinical activity before the Parkinson’s candidate.
Matthias Alder: So, with that program we are now in in late three clinical development we’re going through with the toxicology studies animals studies that are required before we are allowed to take that products into humans that work is progressing while we’re on track to us start the uh to make the direct required filings for start the phase one study in the middle of the year and then take it into the clinic from there on so it’s well in hand and well progressing well.
Matt Pillar: Good and as you as you plan that clinical activity you know you just mentioned part of your work with Michael J fox foundation question is around discovery of biomarkers and other things that indicate to me that perhaps there was an application there’s an application potential for computational approach approaches machine learning or might be on the clinical side as well you know that’s efficiency.
Matthias Alder: Yes
Matt Pillar: Patient population identification you know plenty of outpatients there probably and you and I spoke briefly about this not too long ago, so I want to give you an opportunity to talk about what you envision or intend to do with these high-tech approaches
Matthias Alder: Right
Matt Pillar: As you move from you know development into clinical.
Matthias Alder: And that’s a thank you for raising that’s a very important aspect obviously being a company rooted in on the computational side with our drug discovery platform, we are acutely aware and interested in in exploiting the these advances that really help accelerate or streamline or special make increase the likelihood of success of a clinical program and so we’re looking at a number of platforms that other companies have developed many of them using AI, big data to really source out some of these disease aspects and that allows us maybe potentially to select, have a better patient selection in our clinical studies to ensure that we have the appropriate patients for the product that we are developing. The other aspects include looking at some more hardware like tools that I can’t play a real role in terms of wearable’s looking at symptom progression or lack thereof in the clinical study in Parkinson’s in particular, there’s now you know wristbands and things like that before ongoing measurements in terms of the locomotion impairment, the gait and things like that that we can apply in our clinical study to capture that data and make sure that we’re making the right choices as were developed in developing the product in these patients.

Matt Pillar: Yeah, very cool very exciting of an opportunity again an opportunity for game to be on and uh the leading some of the applications for that new technology very good, all right we’re uh we’re kind of running short on time here, uh with this but I got I Public relations people holding back but when I reflect on what Gain is today what it’s come from and what it’s become today. I see this company that’s built up form that in and of itself is a is a valuable, you know product right to the life sciences community to the to the pharmaceutical biopharmaceutical community and a lot of companies that are trying to do that you know check that CTX platform that you’ve developed and you can just say that’s what we do you know we offer that as a service even to you know to other pharmaceutical companies but I also see a company that’s developed a pipeline and is making you know significant preclinical progress as planned is it going to the clinic next year and I know that there are multiple answers to this question but what’s the end game for you know Matthias’s vision for gain therapeutics do you want to you know do you want to be a service provider slash therapeutic developer do you want to be a you know three to early be clinical developer and then you know sell off assets do you want kind of develop stuff that goes all the way to the commercial finish line like what sort for the we always talk about and you know begin with the end in in mind is there an end in mind for Gain.
Matthias Alder: Some tongue in cheek I would say. You said it to all of the above and that’s the way the way I think about Gain is truly we have two asset pockets that we can leverage one is the pipeline that we have generated with the platform and there we have a lead program in Parkinson’s he’s moving into the clinic we have polo on programs and that I expose the opportunity for intrinsic value creation by the developing these products and creating more evidence that they will actually work and creating more investor confidence. They also provide an opportunity for partnering so as a small company it’s very clear that we are not never ever going to be able to develop all nine programs in parallel and take them all the way to market and so we are very active in looking at engaging with potential partners in the Pharmaceutical to take licenses, engage in collaborations with us which can help us generate no that would give cash inflow for us from licensed revenues and things like that so we’re looking at the pipeline from both angle and the same really for the platform. The platform allows us both to generate additional programs early on that that are actually it’s incredibly inexpensive for us to develop the its initial programs because the computational methods are already there we need just need to rent some supercomputing time and run them and we can come up with new programs in a split second almost, but we’re also looking at the platform from the bank in terms of this it should be valuable and is valuable to you know the Pharmaceutical industry to potential partners who may want to engage into in discovery collaborations with us if they have a protein target of interest that they haven’t been able to find anything to work with we can actually take that protein on our platform and spit out that the other end a product that then that the partner can take on take back and develop themselves and so again there it’s in inherent value creation as well as well as value creation through collaborations and we’re pursuing you know all of these aspects in parallel so for my perspective I’m over incredibly well positioned with the company with gain, to take this take us forward and through this very turbulent time that we now see that capital markets and that has an impact all across the industry but with the assets that that that we have the opportunities of value creation that we have I’m very optimistic about what we can achieve with our team here at Gain in terms of progressing company to which to the next level.
Matt Pillar: Yeah, well-done response to that question because it was thorough yet vague open-ended enough off to keep on PR and IR police have pretty, so thank you whatever I asked you Mattie is that uh is central to the story what are what you know what should I have asked you that I did not ask you.
Matthias Alder : Well I to me we’re uh in the biotech industry and I mentioned that uh before at the reason we’re here and the reason we’re doing what we’re doing is to develop new therapies for patient think patients in need and we haven’t really spent a lot of time talking about that or Parkinson’s program but we are really excited about what we have there because with our approach we are essentially um correcting an enzyme that is the protein in the body that’s not working right and because we are correcting that enzyme right to restore the healthy cell so it’s a very sort of uh fundamental approach that we are pursuing so we’re creating with our with our product we’re creating a healthy cell which then that cell provides longer and that happens specifically also with dopaminergic neurons so these are cells that produce dopamine and the lack of dopamine in the brain ultimately causes I have Parkinson’s symptoms and so with our approach were able to right store the function of the enzyme and the and every store that healthy selling store every store the survival of the cell and as a result restored the production of dopamine in the brain and with that we have the ability to have a disease modify in Parkinson’s patients that currently only get their symptoms treated and eventually be they get worse and worse and worse overtime yeah and so having the opportunity here with getting to be at the forefront in a very severe and significant disease in for doing so significant more for this day needs that’s what it that’s what it’s all about and that has us you know getting up every morning and going to work for patients to provide that benefit um and look forward to that
Matt Pillar: Yeah
Matthias Alder: The coming years to achieve to achieve that big goal.
Matt Pillar: Yeah, definitely and you know I guess it’s important to point you probably already did but just to sort of reinforce force that act that activity that happens at the enzymatic level is post like it’s done without any genetic modification it’s after the genes and events work so I think I think I mean you know I’m not necessarily and observe you know I talk to a lot of folks but it occurs to me that the potential safety advantages and also complexity the advantages over you know perhaps some of the genetic therapies true you know true gene therapies other that are looking at this indication. Yeah, it occurs to me that there are probably some advantages on the Gain’s approach.
Matthias Alder: There are and there is it’s obviously with the only ones developing uh you know new blacks in Parkinson’s disease gene therapy is out there are a number of challenges with these approaches and you know there’s a number of high profile, you know failure shall we say in terms of gene therapy programs. The other aspect is that gene therapy ultimately is always going to be a very expensive therapy and especially for larger indications like Parkinson’s disease, it’s not quite demanded imaginable how any healthcare system can actually really afford paying that therapy for patients who need it and so having an approach like we have which is a small molecule approach is standard way to develop its standard way to manufacture it standard way to commercialize it we’re not trying to forge a new path here it’s going to come out with an effect that is can be gene therapy like in that we’re fixing the enzyme that that gets misfolded because of a genetic mutation so we’re fixing the enzyme and have the opportunity to have that disease modifying effect that you in therapy also has the potential to have and so it’s a great opportunity I think we’re competitive really well positioned we’re clearly the leading program with our mechanism of action and it’s our job to push it through and make it make it that the you know.
Matt Pillar: Yeah,
Matthias Alder: Fulfil the promise that we currently holding our hands.
Matt Pillar: Sure, yeah you your job now but you got the you took the CEO role to come down and
Matthias Alder: Happy to take it on and there and bring it forward bridge absolutely
Matt Pillar: Well, that’s excellent Matthias I really enjoy talking with you, I appreciate you coming on to through the story with us it’s a fascinating work. I think there’s a super insightful for our audience I love these application stories around computational I appreciate the detail you gave us so thank you for joining us and
Matthias Alder: uh you know thank you Matt I’ll make sure we stay in touch and we’ll do it again sometime wonderful much appreciated thanks for the opportunity great to talk to you.
Matt Pillar : Yeah you too so .That’s game therapeutic CEO Matthias Alder I’m Matt and this is the business of biotech, in case you missed it we just launch the newsletter to accompany this program and I’d like you to sign up for it at bio processonline.com\ Bob I’d also like you to show sativa some love for its support of this project by going to cytiva.com\ emerging biotech to check out all the great resources they’ve procured for new emerging biotech in the meantime be sure to subscribe and leave us to review.


